Journal of
eISSN: 2572-8466


Review Article Volume 10 Issue 2
Department of Bioengineering, University of California Los Angeles, USA
Correspondence: Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600,USA
Received: April 14, 2023 | Published: April 25, 2023
Citation: : Squires R, Wu B, Tawil B. Fibrin sealant as a delivery vehicle for cells, antibiotics, growth factors, and painkillers. J Appl Biotechnol Bioeng. 2023;10(2):56-64. DOI: 10.15406/jabb.2023.10.00328
Fibrin sealant has vast uses in surgical settings for both cellular and noncellular delivery. Some advantages include biocompatibility, ability to support cell attachment, and controlled degradation rate. There are many clinical applications, from wound healing to improving bone defects to being used as an adjunct to surgery. It can also serve as a suitable delivery vehicle for cells, steroids, antibiotics, growth factors, chemotherapeutic agents, and painkillers. The composition of fibrin sealant can be altered to allow for controlled release, making it an attractive delivery system. Lastly, a wound healing model with 2 defects in the collagen construct may serve as a future application that utilizes fibrin sealant as a delivery system. This review highlights different uses of fibrin sealant as a delivery vehicle for cells, steroids, antibiotics, chemotherapeutic agents, growth factors, and painkillers.
Keywords: fibrin sealant, cell, steroid, antibiotic, chemotherapeutic agent, growth factor, painkiller
MBCP-FS, macroporous calcium phosphate combined with fibrin sealant; hMSCs, human mesenchymal stem cells; MI, myocardial infarction; PR-FG, platelet-rich fibrin glue; BM-MSCs, bone marrow mesenchymal stem cells; CT, computed tomography; ASCs, autologous stem cells; BAE, bleb associated endophthalmitis; MRSA, methicillin-resistant Staphylococcus aureus; FG-VC, fibrin glue with vancomycin; AFS, fibrin sealant with antibiotic; FGF-2, fibroblast growth factor-2; NGF, nerve growth factor; KGF, keratinocyte growth factor; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; OXP, oxaliplatin; NS, normal saline; CHIC, cisplatin hyperthermic intraperitoneal perfusion chemotherapy; AGC, advanced gastric cancer; FS, fibrin sealant; BPC-MPs, bupivacaine-loaded polylactic-co-glycolic-acid microparticles
Impact statement: While there are many review articles about fibrin, this is the first recent review article about the use of fibrin for the delivery of cells, growth factors, pain killers, antibiotics in research and clinically
From cell delivery to drug delivery, fibrin sealant has many uses in surgical settings. Fibrin sealant has been approved by the Food and Drug Administration as a hemostat, adhesive, and sealant, with many advantages over older treatments.1 Some of these earlier synthetic materials have limitations, including the inability to support cell attachment, biocompatibility concerns, and undesirable degradation rate.2–4 Fibrin sealant is prepared by mixing concentrated fibrinogen solutions with thrombin, which provides the surgical seal, the tensile strength, and the adhesion strength of the clot.1,5-6 When the fibrinogen and thrombin solutions are mixed, the final stage of the coagulation cascade is mimicked.7 This forms a stable clot, which is then naturally degraded by enzymes in the fibrinolytic system and conveniently absorbed by the body during the healing process.7–9 With fibrin sealant, the formation of a stable clot is sped up, the amount of blood lost is decreased, and the risk of infection is reduced.10 The composition of the sealant can be varied to suit a specific need when different degradation rates are required.11 Fibrin sealant has become a valuable adjunct to surgery due to its ability to assist in wound healing and reduce post-operative pain.1,12-17
When reducing pain at the surgical site, fibrin sealant is applied alone or loaded with drug. Because initial pain can be largely attributed to surgical excision and bleeding, the use of fibrin sealant allows for improved pain management.12,13 In trials studying its efficacy, fibrin sealant was found to reduce post-operative pain and bleeding, while also improving quality of recovery.12-17 Additionally, fibrin sealant can be loaded with painkiller when being applied to a wound site.11,14,17,18
In this review, we provide an overview of the use of fibrin sealant in surgical settings, specifically as a delivery vehicle. Topics include the use of fibrin sealant on wound sites and fibrin sealant as both a cellular and noncellular delivery vehicle. We aim to provide a reasonably comprehensive review of the benefits and downfalls of fibrin sealant as a delivery system in research and clinical applications.
Clinical applications of fibrin sealant
When applied to a surgical site, fibrin sealant can serve to both seal the wound and decrease pain levels. Fibrin sealant is a non-cytotoxic and fully resorbable biological matrix that forms a stable clot.8,19 Due to fibrin sealant’s ability to control bleeding at the excision site, it may allow for improved pain management and is a common choice for sealants in various surgeries.1,5,20
Surgery
Gross et al.,12 studied the efficacy of fibrin sealant in reducing post-operative pain for tonsillectomy surgeries. This randomized double blind study was performed on 20 children aged 5 to 17 years old. Pain levels were evaluated at regular intervals for three days following surgery, where fibrin sealant was found to significantly reduce pain and improve quality of recovery after pediatric tonsillectomy. Similar studies analyzing pain levels following tonsillectomy surgeries often reached the same result.15,16,21 Segal et al.,22 is an exception to the above trend, as this study found that the sealant had no significant beneficial impact on post-tonsillectomy pain control, though no adverse effect was found.
In a study performed by Bektas et al.,23 the hemostatic efficacy of fibrin sealant after hepatic resection was investigated. Patients were treated with either fibrin sealant or manual compression on the surgical site. Hemostasis rates of the fibrin sealant group was greater than the control group, while post-operative rebleeding and transfusion requirements were found to be similar. The results demonstrated that fibrin sealant was safe and superior to traditional methods for the treatment of hepatic resection. Fibrin sealant has also been used in hernia repair, where the sealant led to a low rate of hernia recurrence, avoided tissue trauma, and improved pain management.24 From its uses in surgery, fibrin sealant has been found to be effective in improving pain management and quality of recovery.25,26
Wound healing
Healy et al.,27 investigated the use of fibrin sealant in reducing split skin graft donor site pain. Twenty patients received the fabric dressing alone, while the remaining twenty received the fabric dressing plus fibrin sealant at the donor site. Those receiving the fibrin sealant treatment reported significantly less pain and incapacity. Fibrin sealant is a common choice as a sealant on wound sites, due to its ability to both control bleeding and reduce pain. A similar study performed by Mittermayr et al.,28 verified the above findings, and recommended the use of the sealant as a method for skin graft fixation.
Bone defects
Fibrin sealant can be applied to bone defects as a bone graft substitute. In one such study, Wagner et al.,29 studied the formation of new bone after a sinus lift. Macroporous calcium phosphate combined with fibrin sealant (MBCP-FS) was applied to one sinus in patients who required treatment for delayed dental implant placement. The study concluded that MBCP-FS was safe and effective in supporting bone regeneration. D’Arc et al.,30 carried out a similar investigation looking at calcium phosphate loaded with fibrin sealant for mastoid reconstructions in middle ear surgery. CT-scans and analysis of clinical results over 15 years confirmed stable bone reconstruction in the mastoids. Fibrin sealant is an effective adjunct to orthopedic and trauma surgery31 and has many features that make it attractive as a delivery vehicle, which will be discussed in the following section.
Fibrin sealant as a delivery vehicle
Fibrin sealant has various properties that allow it to act as both a sealant and as a delivery vehicle. Through interactions between the gel and cells or other factors, fibrin sealant can seal a surgical site.32 The sealant can also be loaded with drug, cells, painkillers, or other components due to the crosslinking that occurs when fibrinogen and thrombin are mixed.32 Fibrin sealant then becomes an attractive delivery system, as a drug or cells can be added to either the fibrinogen or thrombin component and distribute throughout the solution.32 Additionally, the concentration of fibrinogen and thrombin can be modified to alter the structure, mechanical properties, and degradation rate of the gels.11,33 The next sections will address how fibrin sealant lends itself to being an attractive delivery vehicle for drugs or cells, and can be even more effective for painkillers.34
Research applications
Fibrin sealant has shown to be an optimal delivery vehicle for cells, as it is biocompatible, bioresorbable, and allows for sustained release.34 Cells, specifically human mesenchymal stem cells (hMSCs), have found success in fibrin sealant delivery systems.35,36 These cells are viable in all fibrin concentrations, but have different proliferation rates with different fibrin formulations.35 Because the concentrations of fibrinogen and thrombin can impact the 3D fibrin clot structure and cell proliferation, the formulation chosen must be carefully considered.35,37–39 Growing Cells in 3D Constructs as shown in Figure 1.
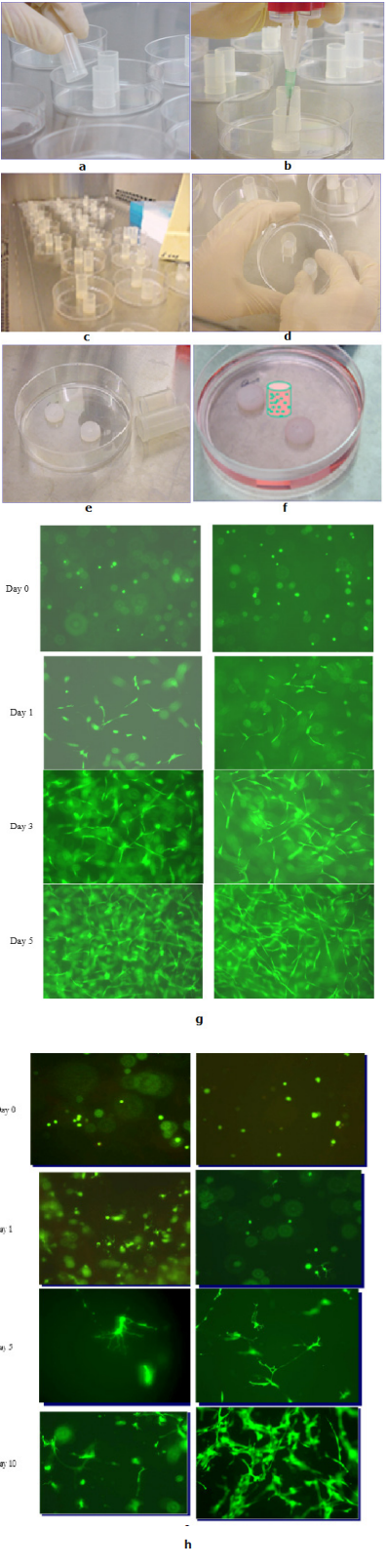
Figure 1 Growing Cells in 3D Constructs; (a) Sterilized Tubes with Open Ends are placed on a Culture Dish; (b) Fibrin Sealant Containing Fibroblasts is Added to the Tubes; (c) Clots are Allowed to Polymerize; (d) Tubes are Removed; (e) Dime Shape Fibrin Clots; (f) Fibrin Clots are submerged with Medium. (g) Growing MSC in 3D Constructs; (h) Growing NSC in 3D Constructs. (h) Growing NSC in 3D Constructs.
Keeping these factors in mind to ensure high cell viability in fibrin, Martens et al.,40 studied the use of biocompatible hydrogels polymerizable in situ as a cell delivery vehicle. Fibrin glue loaded with bone marrow-derived mesenchymal stem cells was used to improve cell retention, survival, and function following delivery into the ischemic myocardium. By controlling the concentrations of the fibrinogen and thrombin components, a fibrin hydrogel that is compatible with current percutaneous injection catheters can be prepared. Although this was only a pilot study, the fibrin sealant delivery system was found to increase the efficacy of cell transfer for cardiovascular disease. In a similar study investigating cell delivery for heart disease, Christman et al.,41 injected isolated myoblasts that were suspended in fibrin gel precursor solution into a damaged heart after myocardial infarction (MI). Fibrin gel increased the survival rate of transplanted cells, increased blood flow, and decreased infarct size when compared to the traditional method of direct injection of cardio myoblasts. Various studies have found similar conclusions that fibrin sealant has no negative effect on cell adhesion, proliferation, or differentiation.42–44
Clinical applications
Falanga et al.,45 investigated how mesenchymal stem cells (MSCs) delivered in a fibrin spray accelerated healing in cutaneous wounds. Autologous MSCs were harvested and loaded into the fibrinogen component of the sealant, which was administered as a spray. Pain relief was substantial in both wounds that had been treated with fibrin alone or fibrin with cells. The wounds healed quickly and the closure was durable. The use of fibrin spray loaded with cell suggested that there may be accelerated healing of the wound.
Platelet-rich fibrin glue (PR-FG) can also be used to deliver bone marrow mesenchymal stem cells (BM-MSCs) as shown by Haleem et al.,46 In this study, PR-FG loaded with BM-MSCs were transplanted onto femoral condyles cartilage defects of 5 patients. After one year, all of the patients’ symptoms improved, and 3 patients had complete defect fill. Based on these results, PR-FG as a scaffold for BM-MSCs is an effective approach in the repair of cartilage defects.
More studies have investigated the use of fibrin sealant in delivering MSCs or autologous stem cells (ASCs), finding positive results when it is used to repair rotator cuffs,47 osteoarthritic knees,48 or perianal fistula.49 All studies found that fibrin sealant was an effective scaffold for the delivery of cells, improving wound repair and enhancing recovery. Because of its efficacy in cellular delivery, the next area that begins to emerge is noncellular delivery via fibrin sealant.
Research applications
Because fibrin sealant has been widely used in various medical settings as well as having good tissue compatibility, it became a clear candidate as a potential drug delivery vehicle. When used as a delivery system, fibrin sealant allows for sustained release and establishes higher local concentrations of the drug, with no negative impact on the drug itself.50,51 Lee et al.,52 discussed the use of fibrin sealant in trans-scleral delivery of dexamethasone, a steroid that treats inflammation. Fibrin sealant provided a uniform release over the course of 24 hours, demonstrating its potential use as a delivery system to sustain drug levels over the posterior segment of the eye. Cruysberg et al.,53 performed similar studies investigating delivery of dexamethasone across the sclera and came to analogous conclusions that fibrin sealant provided a more gradual and uniform release.
Clinical applications
Hayat et al.,54 discussed a case report of a 66 year old woman with delayed onset bleb associated endophthalmitis (BAE). This bleb leak that had followed glaucoma surgery was successfully treated with vitrectomy, intravitreal antibiotics and steroids, intracameral air, and fibrin sealant. After vitrectomy was completed, injection of various antibiotics and the steroid dexamethasone was administered. Fibrin sealant was then applied over the bleb to seal the area. After a follow up with the patient, vision was restored and there was no recurrence of the bleb leak. Based on these results, fibrin sealant and steroid may be used together to manage these leaks, and there may be more clinical applications of this combination in the future.
Research applications
Fibrin sealant can be an effective delivery vehicle that promotes continuous antibiotic release. Shin et al.,55 investigated different concentrations of vancomycin impregnated fibrin sealant. The results found that the peak release of vancomycin was at day 2, with sustained release lasting for 2 weeks, suggesting that fibrin sealant was an effective delivery vehicle for this antibiotic. Another investigation of vancomycin release from fibrin sealant is discussed by Ozaki et al.56 Through animal experiments, fibrin was used as a slow, controlled release delivery system against methicillin-resistant Staphylococcus auereus (MRSA) infection following artificial implantation of prosthetics. Subcutaneous pockets in rodent backs were filled with either fibrin glue alone or with fibrin glue loaded with vancomycin. After the wound was closed, MRSA was then injected. A single dose of FG-VC was effective in sustained release of the antibiotic, protecting against MRSA infection. Because of these conclusions, fibrin sealant proved to be an effective sustained delivery vehicle of antibiotic. Similar studies involving vancomycin via fibrin delivery reached results analogous to those above.57
An in-vitro study performed by Tan et al.,58 investigated the antibacterial effect of teicoplanin antibiotic saturated fibrin sealant against MRSA. Various concentrations of teicoplanin were implanted in fibrin sealant disks, with the higher concentrations maintaining their effect for up to 28 days. The results of this study suggested that fibrin sealant may be effective in delivering antibiotic to local wound infection sites. Fibrin can also be used to deliver antibiotics to orthopedic surgical sites. In a study performed by Cashman et al.,59 an animal model was used to examine how fibrin sealant could deliver antibiotics and reduce infection. Antibiotic loaded fibrin reduced swelling, bacterial count, and infection when compared to rats treated with the antibiotic alone. Similar studies reached analogous conclusions that fibrin sealant was an effective delivery vehicle for antibiotic, allowing for sustained or controlled release and lowered infection rates.11,60–65
Clinical applications
Tofuku et al.,66 investigated the use of fibrin sealant loaded with antibiotic (AFS) in preventing surgical site infections that are associated with spinal instrumentation. At the conclusion of the clinical study, patients that had received an application of AFS has no sign of surgical site infection, demonstrating that when AFS is applied to spinal instrumentation, there are good clinical outcomes in preventing post-operative spinal infections. Singer et al.,67 investigated the use of fibrin sealant in combination with antibiotics for the treatment of fistulas. Patients were broken into three groups: fibrin sealant with antibiotic, fibrin sealant with fistula opening closure, or fibrin sealant with antibiotic and fistula opening closure. Patients were then monitored for a mean of 27 months. The final healing rates of the three groups were 25% for the fibrin sealant with antibiotic group, 44% for the fibrin sealant with fistula opening closure group, and 35% for the combined group. The results showed that none of these groups were more successful than the historic methods of fibrin sealant alone; however, the use of fibrin with antibiotic had no significant negative impact on the patient.
Research applications
Fibrin sealant’s ability for controlled release can also be used in delivery of growth factors, which are central to wound healing and cell proliferation.68 Ishii et al.,69 investigated the use of fibroblast growth factor-2 (FGF-2) in repairs of articular cartilage. Defects in the femoral trochlea of rabbit knees that were too large to undergo spontaneous repair were filled with fibrin sealant loaded with FGF-2, and the release kinetics and bioactivity of the growth factor were studied. Within 24 hours, 50% of FGF-2 was released from the sealant while maintaining its original bioactivity. At the study’s conclusion, the results demonstrated that fibrin sealant loaded with FGF-2 was successful in inducing healing of the cartilage eight weeks after creation of the defect.
Chunzheng et al.,70 investigated the effect of nerve growth factor (NGF) loaded fibrin sealant on peripheral nerve regeneration. Rats were injected with either fibrin glue with NGF, NGF alone, fibrin sealant alone, or normal saline. Results demonstrated that the fibrin glue embedded NGF group had several advantages when compared to the NGF administered alone: fibrin glue delays NGF degradation, it can bind to the nerve because of its adhesive properties, its biodegradability allows for complete absorption by tissue, and it is easy to apply. Additionally, for wound healing, fibrin has found success as a delivery vehicle for Keratinocyte Growth Factor (KGF) and Epidermal Growth Factor (EGF).71,72 Overall, fibrin sealant was found to be an effective carrier for prolonged release of growth factor, and other studies have reached similar conclusions.73–75
Clinical applications
Kipshidze et al.,76 discussed a case report in which fibrin sealant was used to deliver vascular endothelial growth factor (VEGF). The patient, a 66 year old man with moderate to severe ischemia in his toes, was deemed appropriate for angiogenic treatment with fibrin sealant. To perform this procedure, a dual syringe system with the fibrin glue and VEGF was injected close to the popliteal artery. Post-operative follow ups confirmed that the patient’s exercise tolerance increased and pain decreased. Four months after treatments, there was a substantial growth of new vessels at the site where VEGF and fibrin had been injected. Because fibrin sealant allows for sustained release of the growth factor at the target site, this delivery system may have great clinical applications. Other studies reached similar results in that fibrin sealant can act as a scaffold for growth factors.77 Making Blood Vessels as shown in Figure 2.
Research applications
Another application of fibrin sealant is in the delivery of chemotherapeutic agents. Hu et al.,78 investigated the delivery of oxaliplatin (OXP), a chemotherapeutic agent used in colorectal cancer treatment, via fibrin sealant in mouse models. When OXP is combined with fibrin sealant, there is an enhanced anti-tumor performance and decreased proliferation when compared to OXP alone. These findings parallel investigations of fibrin sealant in combination with other chemotherapeutic agents, further confirming that fibrin sealant allowed for sustained release of drug.79–87
Clinical applications
Fibrin sealant delivery of chemotherapeutic drug has also found great clinical success, as it prevents bleeding from the administration site and remains where injected due to its adhesion properties.88 Huang et al.,89 studied the delivery of the chemotherapeutic cisplatin via fibrin sealant compared to traditional cisplatin hyperthermic intraperitoneal perfusion chemotherapy (CHIC) for advanced gastric cancer (AGC) patients. The pharmacokinetic profile of the FS group with cisplatin was more favorable, with a much longer elimination half-life of the drug. After a 40 month follow up, overall survival of the FS group was higher than the CHIC group, demonstrating fibrin sealant’s ability to be an effective and safe delivery vehicle for chemotherapeutic agents.
Opitz et al.,90 investigated the use of fibrin loaded with cisplatin for surgery of malignant pleural mesothelioma. Twelve patients were treated with escalating doses of the cisplatin-fibrin matrix with overall survival of 21 months. The authors concluded that the use of cisplatin-fibrin is safe, and further studies should be carried out. In a case report of cisplatin mixed with fibrin glue for advanced pancreatic carcinoma, this matrix was found to be an effective treatment option for pancreatic cancer.91
Fibrin sealant loaded with painkiller
Many studies have demonstrated fibrin sealant’s ability to be a successful drug delivery vehicle, as mentioned in the previous section, and this opens the door to improving other aspects of surgical care, like delivering pain killers to wound sites. Because fibrin sealant alone can reduce post-operative pain, the gel in combination with painkiller may yield an extremely effective delivery system.11,14,17,18
Research applications
Local injection of painkillers in postoperative pain management often has only short-term efficacy. To overcome these issues Kim et al.,92 proposed a formulation of bupivacaine-loaded poly(lactic-co-glycolic-acid) microparticles (BPC-MPs) in fibrin sealant. In-vivo studies evaluated the pain relief efficacy of the composite fibrin sealant system in pain induced animals, where paw withdrawal indicated pain levels. The fibrin glue group with BPC-MPs exhibited sustained release of bupivacaine for 35 days, and pain relief efficacy was improved compared to other administration methods of the painkiller. Based on these findings, fibrin sealant was deemed an effective delivery vehicle for anesthetic.
Clinical applications
Zhibo et al.,93 discusses the use of fibrin sealant as the delivery vehicle of lidocaine, an anesthetic, after breast augmentation. Post-operative breast pain reported by patients who had received the lidocaine dissolved in fibrin sealant was significantly less than patients who had received either lidocaine or fibrin sealant alone. Fibrin sealant was also able to produce a sustained release of lidocaine, and because fibrin glue only remains in the body for about 1 to 2 weeks, the mixed glue and painkiller did not cause a foreign body reaction. Kitajiri et al.,14 also investigated the use of fibrin sealant loaded with lidocaine and found analogous results. Other combinations of fibrin sealant with painkiller have demonstrated its efficacy as a delivery vehicle, with positive trends in pain management.18,94,95 Similarly to when fibrin sealant is loaded with drug, fibrin sealant loaded with painkiller is an effective sustained release system that does not have a negative impact on the drug itself.
Fibrin sealant is an extremely effective delivery vehicle. The gel is biocompatible, tissue adhering, and biodegradable.1 It has the ability to hold drug adjacent to target tissue and allow for sustained release.11 Currently, some clinical applications of fibrin glue include its use in surgery,12,15,16,23–25 wound healing,27,28 and bone defects.29–31 It has been effective in delivering cells,36–49 steroid,51–54 antibiotic,55–67 growth factor,68–77 chemotherapeutic agent,78–91 and painkiller.11,14,17,18,93-95 Additionally, due to the glue’s ability to reduce pain by controlling bleeding at a wound site, it becomes even more effective when loaded with painkiller. Research efforts are uncovering more potential uses of this material, leading to the growing number of fibrin sealant’s clinical applications. In some cases, the use of the sealant has shown to be more effective than traditional methods of treatment.14,66,89 In the future, a potential application of fibrin sealant may have it being a combined delivery system, where it can deliver drug and cells all in one place.
Future applications
In the form of micro-beads, fibrin sealant, which allows for controlled release, can also be used as a delivery vehicle for various components that are necessary for wound healing. A future therapeutic model where fibrin is used to heal wound sites could be a collagen construct with two defect sites filled with fibrin.96 Inside the collagen construct, there are monocytes and fibroblasts loaded in fibrin beads, and a layer of keratinocytes on the top of the construct. Mogford et al.,97 tested the use of fibrin sealant loaded with fibroblasts on rabbit wounds. The study found that the loaded fibrin sealant enhanced wound healing and repaired defects. Iyer et al.,98 investigated keratinocyte migration in an in-vitro wound healing model co-cultured with fibroblasts and found that the keratinocyte migration rate increased in the presence of fibroblasts, As shown in Figure 3. Each bead could have a different composition of fibrin, which influenced the diffusivity of molecules from these microbeads. This composition of fibrin can then be optimized for controlled release, and various formulations of fibrin sealant can be chosen for co-delivery.33,99-101 This wound healing model may be a future application for how fibrin sealant can be used to both heal a wound site and act as a delivery system for multiple loaded components.
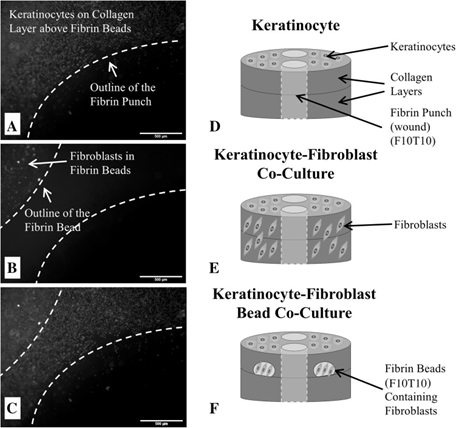
Figure 3 Micrographs of A keratinocytes fluorescently-tagged with Vybrant DiD on the surface of the 3D wound healing construct and near the fibrin-filled defect at a concentration of 300,000 cells/mL; B fibroblasts fluorescently-tagged with Vybrant DiO that are encapsulated inside a fibrin bead within the collagen layers of the 3D wound healing construct and near the fibrin-filled defect (20,000 cells/bead); and C a stacked fluorescent image of the keratinocyte-fibroblast bead co-culture with fibroblasts encapsulated inside fibrin beads. For all micrographs, the scale bar = 500 µm (50 x magnification). D Illustration of the control construct with only keratinocytes seeded on the surface. E Illustration of the keratinocyte-fibroblast co-culture construct with fibroblasts uniformly dispersed throughout the collagen matrix and keratinocytes seeded on the surface. F Illustration of the keratinocyte-fibroblast bead co-culture with fibroblasts encapsulated inside two fibrin beads within the collagen matrix and keratinocytes seeded on the construct’s surface. The final fibrin concentrations were either 5 or 10 mg/mL (F5 or F10) and polymerized with either 5 or 10 units/mL thrombin (T5 or T10). The concentration of the fibrin-filled defect matched the fibrin bead formulation. Note the illustrations are not drawn to scale.89
The authors acknowledge funding support from UCLA.
There are no conflicts of interest presented or declared by the authors in this research.
None.

©2023 :, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.