Journal of
eISSN: 2572-8466


Research Article Volume 10 Issue 1
1Laboratory of Extremophile Plants, Borj-Cédria Biotechnology Center, Tunisia
2Laboratory of Aromatic and Medicinal Plants, Borj-Cédria Biotechnology Center, Tunisia
3Laboratoire LASEVE, Université du Québec à Chicoutimi, 555, Boulevard de l’Universite, Canada 2B1
Correspondence: Oueslati Samia, Laboratory of Extremophile Plants, Borj-Cédria Biotechnology Center, Tunisia, Tel +216 79 325 848, Fax +216 79 325 638
Received: December 16, 2022 | Published: January 10, 2023
Citation: Oueslati S, Feten ZK, Legault J, et al. Antioxidant, anti-inflammatory and anticancer activities of Tunisian halophyte Suaeda mollis. J Appl Biotechnol Bioeng 2023;10(1):1-4. DOI: 10.15406/jabb.2023.10.00318
The aim of this study was to investigate the antioxidant, anti-inflammatory, and anticancer properties of Tunisian Suaeda mollis specie after fractionation by increasing polarity of several extracts (Hexane, Dichloromerthane, Methanol and water). Water and methanol extracts of S. mollis exhibited the best in vitro antioxidant ability (2.92±0.19 and 2.77±0.21 µmol TE/mg, respectively), while, dichloromethane extract displayed the highest ex vivo antioxidant activity with an IC50 equal to 1.73 µg/ml. Besides, hexane extract showed an important anti-inflammatory effect inhibiting NO with 55.9% at 160 µg/ml. Finally, dichloromethane extract displayed a potent anticancer activity against cancerous cells A-549 with an IC50 equal to 70±7 µg/ml as well as non cancerous cells Detroit-551 and WS1 (IC50=81±1 and 62±5 µg/ml, respectively) using resazurin test. Moreover, hexane extract is efficient against Caco-2 (IC50=122 µg/ml). Overall, the results suggested that biological activities are influenced by solvent polarity and extractability.
Keywords: antioxidant activity, anti-inflammatory capacity, Suaeda mollis, anticancer ability
AAPH, 2,2-azobis(2-amidinopropane) dihydrochloride; DCFH, 2’,7’- dichlorofluorescin; ATCC, American type culture collection; CE, catechin equivalent; DCFH-DA, dichlorofluoresceine-diacetate; DMSO, dimethyle sulphoxide; TE, equivalent trolox; DLD-1, Caco-2 and HT-29, human colorectal adenocarcinoma cell lines; A-549, human lung carcinoma cell lines; WS1 and Detroit 551, human skin fibroblast; RAW 264.7, mouse macrophage
Halophyte Plants are a potent source of bioactive metabolites for several agroalimentary, cosmetic and pharmaceutical industries.1 Besides they act as an efficient barrier to increase or/and inhibit reactive oxygen species. These ROS are extremely accumulated under environmental constraints such as salinity and drought.
Besides, Halophytes are considered as a significant pool of natural compounds that can replace and substitute synthetic compounds. For this reason, researches and science have focused on the plants phytochemistry and the identification of natural molecules that are beneficial to human health. Oxidative stress is generated by reactive oxygen species surproduction and is defined as disequilibrium between antioxidant system and reactive oxygen species (ROS) generation. These ROS are extremely toxic leading an irreversible damage to proteins, lipids, DNA and can inducing cell alterations cell.2 In this context, Plants stimulate their antioxidant system in order to alleviate the adverse effects generated after oxidative stress. Nevertheless, antioxidant ability depends on plant species, stress types and duration.3 Besides among these plants, Halophytes manage oxidative stress via an endogenous protective system consisting of enzymatic (ascorbate peroxidase, glutathione reductase, superoxide dismutase, catalase, etc.) and non enzymatic systems (glutathione, vitamins, phenolic acids, flavonoids, tannins, etc.).4 In fact, 80% of these secondary metabolites are attributed for plants. These natural compounds are isolated from several flora species. They act as a potent and an efficient antioxidant to scavenge free radicals by hydrogen atom donation.5 These compounds are known for their broad spectra of biological activities as neuroprotective, anticancer, antimicrobial, hypoglycemic, anti-inflammatory, etc.6 and they have more other functions compared to the synthetic drugs. These secondary metabolites are considered an efficient electron donor, provide as reduction agents, donors of hydrogen and singlet oxygen scavengers.7 Also, polyphenols and flavonoids compounds are involved in the inhibition of hydrolytic and oxidative enzymes.8 This oxidative disturbance may guide to other diseases such as inflammation and cancer. Increased attention has been given to plants which are rich in biochemical constituents and which are considered as natural agents to treat several diseases and to inhibit cancer cells growth.9
In Tunisia, an extensive diversity of edible and medicinal halophyte species used in traditional therapy were equipped with powerful antioxidant system which played an important role in plant physiology adaptation to environmental constraint, among them the edible Suaeda fruticosa exhibits hypoglycaemic and hypolipidaemic activities.10 Suaeda maritima was used as a condiment for salad, and known for their hepatoprotective and antioxidant properties.11 Reaumuria vermiculata was known for antioxidant and anticancer activities.12 The present paper investigates for the first time biological activities of Tunisian Suaeda mollis, although there are many reports for other Suaeda species. This study aimed to assess after fractionation using increasing solvent polarity, antioxidant ability via ORAC and cell based-assays, anti-inflammatory activity and anticancer potentiality of the Tunisian Suaeda mollis.
Plant material and extract preparation
Shoots of S. mollis were collected from Draia (Gabes) (34°13ʹ55ʺN,10°00ʹ49ʺE). In order to assess biological activities, 500g of shoots in powder were extracted in a soxhlet apparatus using different solvents (hexane, dichloromethane, methanol and water) basing on polarity solvents. Then, a filtration was performed, after that, a solvent evaporation under reduced pressure using rotary vacuum evaporator was accomplished, and the residue was dissolved in DMSO ahead of analysis.
Assessment of antioxidant activities
Oxygen radical absorbance capacity (ORAC test)
The ORAC test was carried out in 96-well microplates on a Fluoroskan Ascent FLTM plate reader equipped with a programmed injector. For that, four concentrations of Trolox were used in quadruplicate, and a gradient of 16 doses of the samples was arranged lacking replication. The experiment was realized in controlled condition. A planification of fluorimeter has been assessed to confirm fluorescence of fluorescein each minute after adding 375 mM of AAPH during 35 min. ORAC values were expressed in µmol TE/g which trolox was used as a positive control by Girard-Lalancette.
Antioxidant cell assay using 2',7'-dichlorofluorescin-diacetate (DCFH-DA)
Antioxidant activity was evaluated using the DCFH-DA assay by Legault. Human Skin Fibroblast cells were plated in 96 microwell plates and incubated under controlled condition (5 % CO2 and at 37 °C). After that, phosphate buffer saline was used to rinse cells and an incubation of these cells in Hank’s balanced salt solution with DCFH-DA has been carried out. After that, incubation during 1H of cells in contact with Suaeda mollis extracts was carried out after rinsing the cells. This experiment was carried out with or without tert-butylhydroperoxide. The fluorescence was measured directly after addition of t-BuOOH and after 1h30min at 485 and 530 nm.
Anti-inflammatory activity
RAW 264.7 cells growth was monitored after incubation in culture medium for controlled condition (16h, 37°C and 5% CO2). After that, a treatment of these cells with lipopolysaccharide, extracts and positive control has been assessed. Then, microplates are incubated for 24h in order to liberate NO. The L-NAME was used as positive control.
Following that, supernatant was mixed with Griess reagent and incubated at room temperature for 20 min. Nitrite quantification was revealed using NaNO2 standard curve. The absorbance reading was taken at 540 nm using aVarioskan Ascent.12
Cell culture
The human lung carcinoma A-549 (ATCC #CCL-185), colon adenocarcinoma DLD-1 (ATCC #CCL-221), human colorectal adenocarcinoma Caco2 (ATCC#HTB-37), HT-29 (ATCC#HTB-38) and murine macrophage RAW 264.7 (ATCC #TIB-71) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, USA). The WS-1 (ATCC#CRL-1502), Detroit-551 (ATCC#CCL-110), A-549, DLD-1, Caco2 and HT-29 cell lines were grown in Minimum Essential Medium with Earle’s salts, while the RAW 264.7 cell line was grown in Dulbecco’s modified Eagle’s medium (Mediatech Cellgro®, Herndon, USA). Both media were supplemented with10% fetal calf serum (Hyclone, Logan, USA) for (WS-1, Detroit-551, A-549, DLD-1 and HT-29) but with 20% fetal calf serum for Caco2, solution of vitamins (1×), sodium pyruvate (1×), non-essential amino acids (1×), penicillin (100 IU) and streptomycin (100 µg/ml) (Mediatech Cellgro®). Cells were cultured in a humidified atmosphere at 37°C in 5% CO2.
Cytotoxicity assay
Exponentially growing cells were plated at a density of 5×103 cells per well in 96-well microplates (Costar, Corning Inc.) in 100 µl of culture medium and were allowed to adhere for 16h before treatment. Then, 100 µl of increasing concentrations of extract dissolved in the appropriate solvent (DMSO) were added. The final concentration of solvent in the culture medium was maintained at 0.5% (v/v) to avoid solvent toxicity. The cells were incubated for 48h in the absence or in the presence of extract. Cytotoxicity was assessed using the resazurin reduction test as described by O’Brien et al. (2000). Fluorescence was measured on an automated 96-well Fluoroskan Ascent FlTM plate reader (Labsystems) using an excitation wavelength of 530nm and an emission wavelength of 590nm. Cytotoxicity was expressed as the concentration of extract inhibiting cell growth by 50% (IC50).
Statistical analysis
The values were expressed as means ± standard deviation of three determinations. The results were analyzed by a one-way analysis of variance (ANOVA) and Newman-Keuls multiple test. P values of 0.05 or less were considered as statistically significant.
Table 1 indicated that methanol and water extracts showed the highest antioxidant activity, with ORAC values of 2.77±0.21 and 2.29±0.19µmol TE/mg respectively, followed by dichloromethane extract (1.19±0.07 µmol TE/mg). While activity of hexane extract was less significant. Regarding ex vivo antioxidant ability results presented in Table 1 indicated that dichloromethane extract has powerfully antioxidant activity and inhibited the tBH-induced oxidation of DCFH with an IC50 value equal to 1.73±0.55 µg/ml. Moreover, methanol and water extracts displayed similar activity (IC50 value was about 9 µg/ml). Although, hexane extract showed less oxidation inhibition (IC50 value = 128 µg/ml). These findings demonstrated that S. mollis extracts mainly which are extracted with higher polarity possess an important antioxidant capacity.
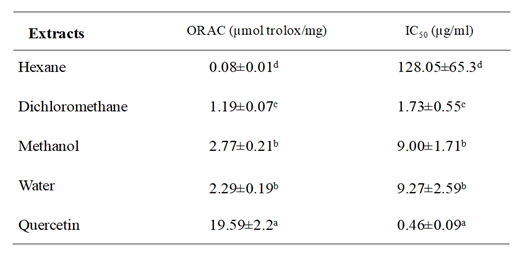
Table 1 Oxygen radical absorbance capacity (ORAC) values and antioxidant cell assay expressed as IC50 of four extracts from Suaeda mollis shoots and standard. Each value represents the mean ± SD of three determinations
Anti-inflammatory activity was evaluated using LPS-stimulated RAW 264.7 macrophages. Stimulation of RAW 264.7 macrophages by LPS induces iNOS (inducible NO synthase) and overproduction of NO. Results presented in Table 2 showed that hexane extract exhibited appreciable inhibitory effect on LPS-induced NO secretion with 55.9 % at 160µg/ml, followed by methanol and water extracts which inhibited NO release by 46.1 and 39.9%, respectively. However, dichloromethane extract showed cytotoxcicity at doses ranging from 2.5 to 160 µg/ml (data not shown). Comparatively, the L-NAME, used as positive control inhibited NO release by 48.7 % at 250.0 µM (67.4µg/ml).
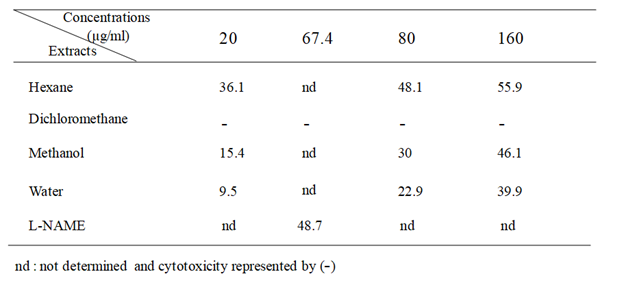
Table 2 Anti-inflammatory activity of several extracts from S. mollis shoots expressed by NO inhibition (%).
Furthermore, anticancer activity of S. mollis has not been shown in literature and the important antioxidant and anti-inflammatory activities in this species related to their phenolics prompted us to investigate their anticancer capacity. The cytotoxicity of the 4 shoot extracts was evaluated against colon carcinoma (DLD-1, Caco-2 and HT-29) and lung carcinoma (A-549) cell lines and health human skin fibroblast cell lines (WS1 and Detroit 551). Results displayed that hexane extract of S. mollis was active against Caco-2 with an IC50 value of 122±4 µg/ml and without any cytotoxicity. Moreover, dichloromethane extract showed an anticancer activity against A-549 and HT-29, although with a cytotoxicity against healthy cell lines (WS1 and Detroit 551) (Table 3). Nevertheless, methanol and water were inactive against all carcinoma cells. Consequently, S. mollis had an influence on viability of cells in hexane extracts that target carcinoma colon cells mainly Caco-2, without cytotoxicity against healthy cell lines.15–28
The extraction, purification and biological activities of secondary metabolites depend on various factors including extraction time, temperature, solvent concentration and solvent polarity by Nawaz. In fact, according to chemical nature, diverse phytochemicals are extracted in solvents using various polarity because the combination of solvents is always effective than the use of single solvents by Iloki-Assanga. Besides, phenolic compound amount increased significantly with high polarity solvent and can rapidly decreased by solvent with higher polarity index such as water by Wakeel. Several studies have demonstrated that bioactivities are dependent on solvent polarity by Herrera-Pool. In fact, change in solvent polarity alters its ability to dissolve a selected group of antioxidant compounds and influences activity estimation.13 Moreover, great intraspecific variations in plant secondary metabolites could be due to changes and fluctuations in soil composition and climate that involve the interaction between plants and the environment and eventually bioactive compounds by Kumar.
Phenolic compounds have been reported to be beneficial in the treatment of chronic inflammatory diseases associated with overproduction of nitric oxide (NO). For instance, previous studies showed that the major compound found in S. mollis extract is rutin also named quercetin-3-rutinoside,14 this compound is known with a wide range of pharmacological properties such as antioxidant activity. Conventionally, it is used as antifungal, an antimicrobial and anti-allergic agent by Al-Dhabi . Conversely, recent investigations have shown several pharmacological benefits to treat various diseases, such as cancer, diabetes, hypertension, and hypercholesterolemia by Sharma.
Besides, 4-hydroxybenzoic, syringic, rosmarinic acids are found in S. molis extracts.14 These compounds have been considered in a number of cancer cell lines at different stages of cancer growth. In this regard, Jin found that rosmarinic acid can prevent tumorigenesis and reduce or inhibit tumor growth. Besides, in this context, the antiproliferative role of rosmarinic acid was exerted through apoptotis induction and arrest of cell cycle at G0/G1 and G2/M phases in oral cancer cells by Luo. A potent impact of rosmarinic acid have been demonstrated against several cell lines such as HeLa, HT29, A549 and MCF6 cancer cell lines by Erenler.
Conclusion
In the present study, biological activities including antioxidant, anti-inflammatory and anticancer potentialities of Tunisian Suaeda mollis shoots are demonstrated for the first time in four different solvents. Results showed that this specie has proven the potential benefits. Shoot extracts were found to be a potent antioxidant source and exhibit an important in vitro and ex vivo antioxidant activity. Moreover, this medicinal halophyte showed an important anti-inflammatory against macrophages. Besides, an efficient specificity on colon cancer has been demonstrated using several cell lines. These data suggest that this halophyte could be considered as a potential source of bioactive compounds with advantageous proprieties, suggesting its use in agroalimentary and medicine.
This work was supported by the Tunisian Ministry of Higher Education, Scientific Research and Technology (LR10CBBC02). The authors would like to thank Catherine Dussault for his help in biological assays and its technical assistance and Pr. Abderrazek Smaoui , the botanist who authenticated specimens of the plant in their native ecosystems and established their taxonomic identification.
The authors declare that they have no conflict of interest.

©2023 Oueslati, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.