International Journal of
eISSN: 2381-1803


Research Article Volume 13 Issue 3
1Department of Zoology, Mizoram University, India
1Department of Zoology, Mizoram University, India
Correspondence: Ganesh Chandra Jagetia, Department of Zoology, Mizoram University, 10, Maharana Pratap Colony, Sector-13, Hiran Magri, Udaipur-313002, India
Received: September 22, 2019 | Published: May 4, 2020
Citation: Jagetia GC, Vanlalhruaii F. Anticancer potential of mimosa pudica linn. Lajwanti in cultured dalton’s ascites lymphoma cells. Int J Complement Alt Med. 2020;13(3):91?94. DOI: 10.15406/ijcam.2020.13.00499
The constant use of chemotherapy induces resistance in the cancer cells and they become refractive to treatment leading to treatment failure. Therefore, it is necessary to screen newer agents which are less toxic and kill neoplastic cells effectively. Plants have provided several anticancer modern chemotherapeutic drugs including vinca alkaloids, taxols, epipodophyllotoxins etc. and still can provided newer molecules for cancer treatment. Therefore, present study was undertaken to study the cytotoxic activity of Mimosa pudica or Lajwanti whole plant extracted in chloroform, ethanol and water in cultured Dalton’s ascites lymphoma cells that were treated with 0, 10, 25, 50, 75, 100, 150, 200 and 250µg/ml of chloroform, ethanol and aqueous extracts by MTT assay. The chloroform, ethanol and aqueous extracts of Lajwanti killed Dalton’s ascites lymphoma cells in a dose related manner and the aqueous extract was most potent when compared to ethanol and chloroform extracts. The aqueous extract had an IC50 value of 71.32µg/ml followed by ethanol extract with an IC50 of 90.33µg/ml, whereas chloroform extract was least effective with an IC50 of 1190.69µg/ml. Our study demonstrates that Lajwanti, a traditionally used plant in Ayurveda has a cytotoxic activity and may be useful to kill neoplastic cells.
Keywords: mimosa pudica, dalton’s ascites lymphoma cells, anticancer, MTT
Cancer is a group of diseases, where a group of cells displays uncontrolled growth, invasion and metastasis.1,2 Lifestyles changes and environmental factors also contribute to the increased frequency of cancer in modern era.3–5 Cancer is one of the leading causes of death and it is second global cause of disease related deaths. Approximately 18 million cancer cases have been diagnosed globally, out of which approximately 4.17million cancer patients succumbed to death.6 This indicates that despite the development and availability of most state of art treatment regimens, cancer mortality has not come down. The constant application of modern chemotherapeutic agents used in the treatment of cancer leads to resistance to these molecules and treatment failure.7
The plants have been used since time immemorial to treat various diseases including cancer and it is known that 75% of drugs in the markets are either derived from plants or plant metabolites have formed the main source for development of these drugs.8,9 The most important of these biologically active constituents of plants are alkaloids, flavonoids, tannins, steroids and phenolic compounds.10–12 The Mimosa pudica (Lajwanti) or Chui Mui is a creeper belonging to family Fabaceae. Ayurveda describes Lajwanti as tikta (bitter), kashaya (astringent) and sheetha (cold) in nature. Lajwanti is analgesic, antidepressant, alexipharmic, antiasthmatic, stimulant, and vulnerary. It is a remedy for asthma, dysentery, leprosy, inflammations, burning sensation, leukoderma, fatigue, blood diseases, vaginal, and uterine complaints. The crushed whole plant is applied in itchiness and itch related diseases.13,14 The roots of Lajwanti are used to treat angiopathy, dysentery, fevers, leucoderma, metropathy, jaundice, ulcers, swellings, small pox, bronchial asthma, and strangury. Its leaves are useful in arresting bleeding, fistulous withers, bleeding piles, hydrocele, cuts, hemorrhoids, pinkeye and scrofula. The complete Lajwanti plant employed in rheumatoid arthritis, dropsy, diarrhea, myodynia, amoebic dysentery (raktaatisaara), and uterine tumors in Ayurveda.12–15 Lajwanti extracted in different solvents has antiandrogenic, antibacterial, anticonvulsant, antinociceptive, antioxidant, antidiabetic, anti-inflammatory, antifungal, antitumor, antiulcer, hepatoprotective, antihyperglycemic, immunomodulatory, antifertility, diuretic, antimalarial, antidepressant, antidiarrheal, and antivenomic activities.12–18 Mimosa pudica leaf decoction has been reported to protect mice against pentylentetrazol and strychnine-induced seizures and it is antagonistic to the N-methyl-D-aspartate-induced turning behavior in mice.19 The oral administration of different doses of ethanol extract of whole Lajwanti has been reported to heal excision wounds in mice in a dose dependent manner.14 The topical application of Lajwanti leaves extracted in ethanol healed incision wound in diabetic rats, whereas chloroform and methanol root extracts healed excision, incision and burn wounds in rats.20,21 The acute toxicity studies of chloroform and methanol extract up to 2g/kg orally did not cause any toxicity in rats.20 Mimosa pudica or Lajwanti has been used for various medicinal purposes however, its cytotoxic effects remained unexplored. Therefore, the present study aimed to study the cytotoxic effects of Mimosa pudica in cultured Dalton’s ascites lymphoma cells.
Chemicals
Fetal calf serum (FCS), L-glutamine, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), and minimum essential medium (MEM), were purchased from Sigma Aldrich Chemicals Private Limited, Bangalore, India. Petroleum ether, chloroform and ethanol, hydrochloric acid, and n-butanol were procured from SD Fine Chemicals, Mumbai, India.
Preparation of extract
Mimosa pudica Linn. or Lajwanti also known as Chui mui belongs to family: Fabaceae The Department of Horticulture Aromatic and Medicinal Plants, Mizoram University, Aizawl, India identified the plant. The plants free from fungal infection were collected from the Mizoram University campus in September to December, which is usually dry. The whole collected plants were thoroughly washed with water so as to remove dust and other extraneous materials and dried in shade before converting them into powdered form by an electrical grinder. Usually 100g powder of Mimosa pudica plant was extracted sequentially with petroleum ether, chloroform, ethanol and water in a Soxhlet apparatus.12,14 All extracts were dried and stored at -80°C before use. Henceforth the chloroform, ethanol and water extracts of Mimosa pudica will be called as MPC, MPE and MPA.
Preparation of drug
The MPC, and MPE were dissolved in DMSO whereas MPA was dissolved in MEM. The amount of DMSO did not exceed 0.01%.
The Tumor cells
The Dalton’s ascites lymphoma (DAL) cells were collected from a tumorized mice (sterilized by wiping the whole body by 70% ethanol in an aseptic condition in a vertical laminar flow apparatus). The DAL cells were treated with ammonium chloride to free them from erythrocytes, washed twice with sterile phosphate buffered saline (PBS) and centrifuged at 1000rpm. The viability of DAL cells was determined by trypan blue dye exclusion assay and usually 5X103 DAL cells were inoculated into several wells of individual microplates (Corning Life Sciences, Gurugram, Haryana, India) and allowed to attach for 24h in a CO2 incubator (Eppendorf India, Chennai). The DAL cells were grouped as follows: -
MEM group: The DAL cells in the microwells were treated with equal amount of DMSO in MEM.
MPC group: The microwells seeded with DAL cells of this group were inoculated with 10, 25, 50, 75, 100, 150, 200 and 250µg/ml of chloroform extract of Lajwanti 24h after cell attachment.
MPE group: The DAL cells in the wells of microplate belonging to this group were treated with 10, 25, 50, 75, 100, 150, 200 and 250µg/ml of ethanol extract of Lajwanti 24h after the attachment of the cells.
MPA group: This group of microwells of a microplate containing DAL cells was treated with 10, 25, 50, 75, 100, 150, 200 and 250µg/ml of aqueous extract of Lajwanti 24 h after cell attachment.
Cytotoxicity determination
The cytotoxic effect of 10, 25, 50, 75, 100, 150, 200 and 250µg/ml of Lajwanti extracted in chloroform, ethanol or water was determined by MTT assay of Mosmann.22 The DAL cells (5X103) seeded into several individual wells of microplates, and allowed to attach for the next 24 h. Next day, cells were washed with sterile PBS and were treated with 0, 10, 25, 50, 75, 100,150,200 and 250µg/ml MPC, MPE and MPA and left in the CO2 incubator for another 48 h. The microplates were removed and DAL cells treated with MTT dissolved in serum-free MEM (1.2mM) for the next 4h. The formazan crystals thus formed were dissolved into lysis buffer containing 12% SDS, 5% isobutanol and 12mM HCl and the absorbance was recorded at 570nm using a microplate reader (SpectraMax M2, Molecular Devices, San Jose, CA, USA). Usually 8 microwells containing DAL cells were treated with DMSO or each concertation of, MPC, MPE or MPA and the experiment was thrice done. The cytotoxicity was calculated as follows: -
Viability% = Absorbance of test sample × 100/Absorbance of solvent control.
Statistical analysis
The statistical significance between the treatments was determined by one-way analysis of variance with application of Tukey’s post-hoc test. The test of homogeneity was carried out to determine the difference among the three experiments. Since the differences among all experiments was non-significant, the results were combined and presented in table and plotted as figures.
The cytotoxic effect of MPC, MPE and MPA represented in Table 1 and figure 1 as mean ± SEM (standard error of the mean).
Cytotoxicity
The DAL cells treated with 10 25, 50, 75, 100, 150, 200 and 250µg/ml. of MPC caused a dose related cytotoxic effect and the greatest cell mortality was observed at 250µg/ml MPC (Figure 1). The significant decline in cell survival was recorded only at 100 μg/ml MPC and thereafter (Table 1). The calculation of IC50 showed that it is 1190.69µg/ml (Table 1). When cells were treated with 10, 25, 50, 75, 100, 150, 200 and 250µg/ml MPE, the cytotoxic effect rose depending on the concentration and a maximum cytotoxicity was estimated at a dose of 200µg/ml (Figure 1). The cytotoxicity was significantly higher at 50μg/ml onwards when compared to MEM treatment (Table 1). The IC50 was determined as 90.33µg/ml. The DAL cells treated with 10, 25, 50, 75, 100, 150, 200 and 250µg/ml MPA also induced cytotoxic effect depending on the MPA concentrations and a maximum cytotoxic activity of MPA was estimated at a dose of 250µg/ml (Figure 1). The cytotoxicity of MPA was discernible at a concentration of 25μg/ml, where it was significantly higher when compared to MEM group (Table 1) However, difference between 200 and 250µg/ml was not significant and the IC50 was found to be 71.32µg/ml for MPA. Despite this fact MPE was most cytotoxic at 200 and 250μg/ml (Table1). The data for all extracts were fitted on second order polynomial equation and coefficient of correlation r2=0.95 was calculated for MPC and MPE whereas it was r2=0.97 for MPA (Figure 1).
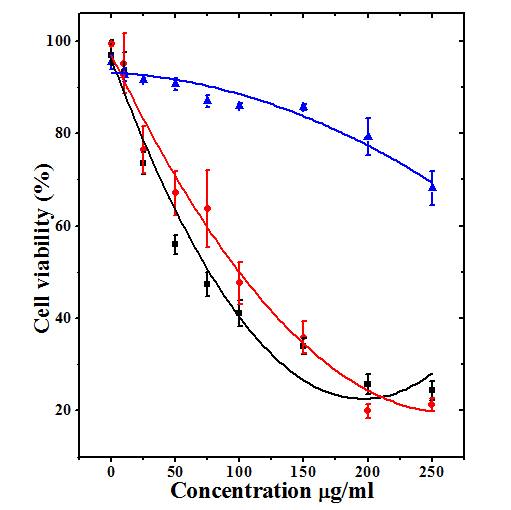
Figure 1 Cytotoxicity induced by various concentrations of chloroform (Triangles; r2=0.95), ethanol (Circles; r2=0.95) and water (Squares; r2=0.97) extracts of whole Mimosa pudica (Lajwanti) in cultured Dalton’s ascites lymphoma cells. N=8.
|
Concentration (µg/ml) |
Cytotoxicity mean ±standard error of the mean |
||
|
Chloroform extract |
Ethanol extract |
Aqueous extract |
|
|
0 |
95.4±1.55 |
99.3±0.41 |
96.9±3.17 |
|
10 |
92.7±1.45 |
95.1±6.52 |
93.1±4.36 |
|
25 |
91.6±0.37 |
76.5±5.07 |
73.5±2.36*** |
|
50 |
86.9±1.24 |
67.0±4.70** |
55.9±2.13*** |
|
75 |
90.6±1.24 |
63.7±8.32*** |
47.3±5.26*** |
|
100 |
86.0±0.62* |
47.5±4.63*** |
41.0±2.78*** |
|
150 |
85.7±0.63* |
35.8±3.45*** |
33.9±1.70*** |
|
200 |
79.2±4.04** |
19.9±1.49*** |
25.6±2.18*** |
|
250 |
68.2±3.65** |
21.3±1.36*** |
24.3±2.03*** |
|
IC50 |
1190.69 |
90.33 |
71.32 |
|
r2 |
0.95 |
0.95 |
0.97 |
Table 1 Cytotoxic activity of different extracts of Mimosa pudica against Dalton’s asctes lymphoma cells in vitro.
*p˂0.05; **p<0.0001 and ***p<0.00001. The significance between the control and different doses of treated groups was tested by one way ANOVA with application of Tukey’s post-hoc test.
Anticancer agents extracted from plant sources have been used for the cancer treatment due to various modes of action on the cancer cells.8,9 Low cytotoxicity to healthy cells and high cytotoxicity to cancerous cell is the ultimate goal in cancer treatment regimes. Herbal medicine is based on the premise that plants contain natural substances that can promote health and alleviate illness. There are several plants which have not been tested for their anticancer activity. Therefore, present study has been attempted to evaluate the cytotoxicity of Mimosa pudica in cultured Dalton’s ascites lymphoma cells.
To evaluate the anticancer potential of any drug 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay serves as a preliminary technique to determine whether any drug or natural product is able to kill the cells efficiently. MTT, a water-soluble tetrazolium salt, gets converted into an insoluble purple formazan by succinate dehydrogenase which cleaves its tetrazolium ring in the mitochondria by live cells. The formazan thus formed is unable to leak through the cell membranes and is congregated in the healthy cells and thus is a short-term assay to detect the cytotoxic effects of any physical or chemical agent. MTT assay was first described by Mosmann.22 The chloroform, ethanol and water extracts of Lajwanti induced cytotoxicity in DAL cells depending on the concentration of the extracts and the aqueous extract of Lajwanti was most cytotoxic as indicated by a lowest IC50 of 71.32µg/ml against the IC50 of 90.33µg/ml for ethanol and IC50 of 1190.69µg/ml chloroform extracts. The tannins extracted from Lajwanti have been reported to be cytotoxic to in vero and MDMK cells in vitro with and IC50 of 0.0653 and 0.119mg/mL.23 The aqueous extract of Helicia nilagirica, ethanol extract of Schima wallichii and Colocasia gigantea have been reported to kill HeLa and V79 cells depending on concentration by MTT and clonogenic assays earlier.24-26 Likewise, Psidium guajava L. leaf oil and Ocimum basilicum L. oil in HeLa cells treated with Cotinus coggygria Scop., Rosa damascena Miller, Colchicum sanguicolle K.M. Perss, and Centaurea antiochia Boiss. var. praealta by MTT assay earlier [28]. showed cytotoxic effect on KB and P388 cells in an earlier study.27 A dose dependent cytotoxicity has been observed
The mechanisms involved in the cytotoxic activity of different extracts of Lajwanti is not clearly understood. However, Lajwanti contains alkaloids, flavonoids, cardiac glycosides, phenols and saponins,12,29 which may have been responsible for its toxic effect on DAL cells. The chloroform extract was least active, whereas the aqueous extract was most powerful followed by ethanol extract due to the variability in the presence of secondary metabolites in these extracts. The Lajwanti extracted in water and ethanol may have caused membrane damage due to triggering of lipid peroxidation and increased lactate dehydrogenase in the DAL cells leading to non-apoptotic form of cell death. In fact, increased lipid peroxidation has been reported to elevate DAL cell death by plant extracts earlier.24-26 This may have been followed by an attrition in the glutathione, glutathione-s-transferase, catalase and superoxide dismutase levels in DAL cells by the aqueous and ethanol extracts of Lajwanti. A decline in all these antioxidants has been observed earlier by aqueous extract of Helicia nilagirica.30 The Lajwanti extracted in water and ethanol may have also induced DNA damage and apoptosis leading to cell death. Earlier plant extracts have been reported to kill DAL cells by inducing DNA damage and apoptosis.24–26 The chloroform, ethanol and aqueous extracts of Croton caudatus have been found to by cytotoxic and ethanol extract was most potent that triggered HeLa cell death by stimulating apoptosis and DNA damage.31 Though we have not investigated the apoptosis and DNA damaging ability of Lajwanti, it is assumed that it would have certainly used these pathways to kill DAL cells. The aqueous and ethanol extracts of Lajwanti may have suppressed the activation of NF-κB and COX-II followed by activation of p53 and caspases leading to killing of DAL cells. Molecular docking studies have revealed that Lajwanti could act as a COX-I and COX-II inhibitor.32 The L-mimosine present in Lajwanti suppressed the inflammatory marker like TNF-α, and IL-1β, which have been reported to be elevated in cancer cells.33,34 The cytotoxic effect of Lajwanti seems to be due the presence of mimosine, tannins and other secondary metabolites.23,28
The treatment of DAL cells with different concentrations of chloroform, ethanol and aqueous extracts of Lajwanti has killed these cells in a concentration dependent fashion and the aqueous extract was most powerful than the ethanol and chloroform extracts. The cytotoxic effect of Lajwanti may be due to increase in DNA damage, apoptosis and lipid peroxidation and suppression of NF-κB, COX-II, TNF-α and IL-1β followed by upregulation of p53 and caspases in the present study. The cytotoxic effect of Lajwanti is due to the presence of mimosine, tannins and other secondary metabolites.
The authors are thankful to the University Grants Commission, Government of India, New Delhi vide Grant No. F4-10/2010(BSR) for providing financial assistance to carry out this study.
Author declares that there are no conflicts of interest.
None.

©2020 Jagetia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.