International Journal of
eISSN: 2381-1803


Research Article Volume 9 Issue 2
Department of Zoology, Mizoram University, India
Correspondence: Ganesh Chandra Jagetia, Professor, Department of Zoology, Mizoram University, Aizawl-796 004, india, Tel 091-389-2330724
Received: March 30, 2017 | Published: November 1, 2017
Citation: Jagetia GC, Lyngdoh R, Lalramchuana, Borah BK (2017) Mimosa pudica (Lajwanti) Accelerates Repair and Regeneration of Deep Dermal Excision Wound in Swiss Albino Mice. Int J Complement Alt Med 9(2): 00293. DOI: 10.15406/ijcam.2017.09.00293
The present study was carried out to evaluate the wound healing potential of the ethanol extract of the whole lajwanti plant on the deep dermal excision wounds in mice. The dried powder of whole plant was sequentially extracted in petroleum ether, chloroform, ethanol and water. Different doses of dried ethanol extract were orally administered in mice for six consecutive days before creation of the rectangular deep dermal excision wound. The wound contraction was recorded daily followed by monitoring the animals for complete healing of wounds. The ability of ethanol extract to influence the collagen, hexosamine, DNA and nitric oxide syntheses and glutathione, catalase, superoxide dismutase and lipid peroxidation was also determined at day7 and 12 post wounding. The administration of 50, 100, 150 and 200 mg/kg body weight of ethanol extract of lajwanti increased the wound contraction in a dose dependent manner accompanied by the reduction in the mean wound healing time which was shortest (18 days) for 200 mg/kg when compared to untreated control (24 days). The ethanol extract of lajawanti increased the synthesis of collagen, hexosamine and DNA and reduced the nitric oxide synthesis on 7 and 12 days post wounding. The glutathione concentration and the activities of catalase, and superoxide dismutase also increased in a dose dependent manner followed by the alleviation of lipid peroxidation. The present study demonstrates that lajwanti enhanced wound healing by increasing the synthesis of collagen, hexosamine, and DNA as well as glutathione concentration and the activities of catalase, and superoxide dismutase enzymes. It also reduced nitric oxide synthesis and lipid peroxidation.
Keywords: mice, mimosa pudica, wound healing, collagen, hexosamine, antioxidants and lipid peroxidation.
The traditional medicine has originated with the infliction of diseases in the human beings as the natural products including plants were in the easy reach of humans. The humans have depended on plants and other natural products for their healthcare since time immemorial. The herbs and natural products continue to play a major role to treat several diseases even during the modern era. According to estimate by World Health Organization 80% of the world’s population including developed and developing countries use plants and natural products for their healthcare.1 The pharmacopeias were dominated by use of plants and natural product 200 years ago. However, the use of plants as medicine declined with the establishment of pharmacology in the therapeutics and development of several chemical synthetic drugs.2 Despite the availability of modern medical system the majority of the word population relies on medicinal plants because plants have been tested for several generations for their use as medicine to treat different ailments and the used of medicinal plants has been considered non–toxic at the dosage that are administered for the treatment of different ailments used. This may be because of their biologic origin in contrast to modern synthetic drugs, which are toxic even at their effective dosages. The importance of medicinal plant can be realized by the fact that usage of herbal medicine has been constantly increasing in the general population of USA and its use increased as much as 380% from a mere 2.5 to 12.1% between 1990 – 1997.3
The toxicological, therapeutic effects of plants and minerals in the Indian subcontinent have its origin in prehistoric times, where plants and minerals have been used successfully to treat various disorders and the Indian system of traditional medicine the Ayurveda is at least 5000 year old and its texts give a good account of various diseases and their treatment. The interest in allopathic medicine also declined due to severe side effects and overuse of the modern drugs. The popularity of the use of traditional medicine among humans has resulted in the resurgence of plants as medicine during the last 20 years. It is also known that many of the modern drugs have their origin in plants before they were chemically synthesized.4 The beauty of plants as medicine lies in the fact that they synthesize several chemicals, some of them may be active as medicine/s, whereas others may act as detoxifying agents when a whole plant product is given in comparison to an isolated compound though may be effective but could produce adverse side effects on human health.5
The wounds are of common occurrence and it will be difficult to find any individual who did not suffer from wound injuries. The wound can result from physical or chemical agents and the wound can be considered as a disruption of the protective function of the skin and a breach in the epithelium with or without the loss of muscle, bone, nerves, etc.6 The wound healing is a complex process that involves continuous cell–cell and cell–matrix interactions. The wound healing proceeds in three overlapping phases: inflammation (0–3 days), cellular proliferation (3–12 days) and remodeling (3–6 months).7–9 It has been reported that only 1–3% of drugs listed in Western pharmacopoeia are intended for use in the skin and for wound repair.10 Both traditional and Western systems of medicine for wound healing suffer from the lack of resources and awareness. Wound healing constitutes a major problem due to the high cost of therapy and the presence of unwanted side effects.11 which indicates the need to find newer paradigms for wound healing.
TheMimosa pudica(lajwanti) is a creeping annual or perennial herb of the pea family fabaceae. The Ayurveda texts describe lajwanti as tikta (bitter) and kashaya rasa (astringent) in taste and by nature it is considered sheetha (cold). Its use balances kapha, and pitta. It is described as antiasthmatic, stimulant, pain–killing and antidepressant remedy in Ayurveda. The whole plant is crushed and used to relive itchiness and itch related diseases. The roots of lajwanti are used to treat leucoderma, angiopathy, metropathy, ulcers, dysentery, swellings, jaundice, bronchial asthma, small pox, strangury, and fevers. Its leaves are useful in hydrocele, hemorrhoids, fistulous withers, scrofula, pinkeye, cuts and bleeds.
The whole plant of lajwanti is used to cure dropsy, rheumatoid arthritis, myodynia and uterine tumors in Aurvedic medicine.12 All parts of this plant are considered to possess medicinal properties and it is used in the treatment of biliousness, leprosy, dysentery, vaginal and uterine complaints, inflammations, burning sensation, fatigue, asthma, leucoderma, and blood diseases.13 Lajavanti has been reported to be active against diarrhea (athisaara), amoebic dysentery (raktaatisaara), bleeding piles, and it also arrests bleeding.12 Different extracts of lajavanti have been found to possess antinociceptive, antiandrogenic, antidiabetic, antibacterial, antiinflammatory, antifungal, anticonvulsant, antioxidant, antitumor, antiulcer, antihyperglycemic, immunomodulatory, antifertility, diuretic, hepatoprotective, and wound healing activities.12,14 It has also been credited to possess antidiabetic, antifertility, antimalarial, antidepressant, antidiarrheal, and antivenomic activites.15 Mimosa pudicaleaf extract has been reported to protect mice against pentylentetrazol and strychnine–induced seizures and N–methyl–D–aspartate–induced turning behavior in mice.16 The acute toxicity studies of chloroform and methanol extracts up to 5 g/kg orally did not cause any toxicity in mice.17 The systematic studies on the use of ethanol extract of whole Mimosa pudica(lajwanti) plant on wound healing are lacking therefore the present study was designed to evaluate the effect of lajwanti on the deep dermal excision wound of mice.
Chemicals
Hydroxyproline (catalog No: H5534), chloramine–T (catalog No: C9887), deoxyribonucleic acid (catalog No: D4522), diphenylamine (catalog No: D2385), ρ dimethylaminobenzaldehyde (catalog No: 42363–0250), thiobarbituric acid (TBA), naphthyl ethylene diamine dihydrochloride (NEDD) and sodium nitroprusside were procured from Sigma Aldrich Chemical Co., St. Louis, MO, USA, while carboxymethylcellulose (CMC), methanol, ethanol, trichloroacetic acid, sodium hydroxide, hydrochloric acid, sodium chloride, n–butanol, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, sodium carbonate, sodium bicarbonate, ethylenediaminetetraacetic acid (EDTA), sulphanilamide, phosphoric acid and potassium chloride were supplied by SD fine–chemicals Ltd., Mumbai, India.
Animal care and handling
The animal care and handling were carried out according to the guidelines issued by the World Health Organization, Geneva, Switzerland and the INSA (Indian National Science Academy, New Delhi, India). Usually, 6 to 8 weeks old healthy male Swiss albino mice weighing 30–35g were selected from an inbred colony maintained under the controlled conditions of temperature (25±2 °C), humidity (55–60%) and 12 hours of light and dark cycle, respectively. The animals were housed in a sterile polypropylene cage containing paddy husk (procured locally) as bedding material. The animals had free access to standard rodent diet and water. All animal experiments were carried out according to NIH and Indian National Science Academy, New Delhi, India guidelines. The study was approved by the Institutional Animal Ethics Committee of the Mizoram University, Aizawl, India vide letter no. IAEC/4503.
Experimental protocol
The wound healing activity of ethanol extract of lajavanti was evaluated in the deep dermal excision wound created on mice dorsum according to the details given below and a total of 300 animals were used to complete all experiments.
Preparation of extract
The plant Mimosa pudica or lajwanti (family: Fabaceae) was identified and authenticated by the Department of Horticulture Aromatic and Medcinal Plants, Mizoram University, Aizawl, India. The non–infected whole plants were collected from the Mizoram University campus during the month of September to December. The plants were cleaned; shade dried and powdered using an electrical grinder. The powdered form of Mimosa pudica plant was sequentially extracted with petroleum ether, chloroform, ethanol and water using Soxhlet apparatus. The ethanolic extract was dried and stored in the refrigerator for further use. Henceforth the ethanol extract of Mimosa pudica will be called as MPE.
The wound healing potential of lajwanti was performed by dividing the animals into the following groups:–
Production of full–thickness skin wound
The full thickness deep dermal excision wound was created on the dorsum of mice as described earlier.18 Briefly, the fur of the dorsum of each animal was removed with a cordless electric mouse clipper (Wahl Clipper Corporation, Illinois, USA). The animals were anaesthetized using controlled anestheisa and the entire body was cleaned and decontaminated by wiping with 70 % ethanol. The cleared dorsal surface of the skin was marked with a sterile rectangular (2.5 x 1.5 cm) acrylic stencil. A full thickness dermal wound was created by excising the full thickness skin flap in an aseptic environment under a vertical laminar flow apparatus using sterile forceps and scissors. Each wounded animal was housed in a separate sterile polypropylene cage until the termination of experiments. The following studies were carried out:–
PI=Sample–Blank/Sample x100
Analysis of data
Statistical significance between the treatments was determined using one way ANOVA and Bonferroni’s post–hoc test was applied for multiple comparisons wherever necessary. The Student’s‘t’ test was used for biochemical estimations. The Solo 4 Statistical Package (BMDP Statistical Software Inc., Los Angeles, CA, USA) was used for data analysis.
The results of wound contraction, mean wound healing time and biochemical estimations are expressed as Mean ± SEM (Standard Error of Mean) in Table "1–2" & Figure 1–10).
Wound contraction
Periodical estimation of wound contraction by recording the wound area provides a correct measure of wound repair and regeneration. Area of each wound at a specific time has been expressed as the percentage of its original size on day one (Figure 1). The mean corresponding area of wound for each group is plotted as a function of days after wounding (Figure 1). Measurements of wound areas regularly revealed that the rate of wound contraction of excision wound progressed with time and the untreated wound completely closed by day 24 (Figure 1). Treatment of mice with different doses of MPE resulted in the enhancement in wound contraction in a dose dependent manner (Figure 1). The wound contraction was almost similar to that of when the animals were treated with the lowest dose of 50 mg/kg until 9 days post–treatment, thereafter the wound contraction was greater and complete wound healing was achieved by day 22. A further increase in the MPE dose to 100mg/kg accelerated the wound healing however, complete closure of wound was achieved by day 22 similar to that of 50mg/kg MPE. When the dose of MPE was increased up to 150 and 200 mg/kg the wound regeneration increased as indicated by a higher wound contraction and the complete closure of wound was affected by 20 and 18 days, respectively (Figure 1).

Figure 1 Acceleration of wound contraction by different doses of ethanol extract of Mimosa pudica (MPE) in the Swiss albino mice inflicted with deep dermal excision wound.
Squares: CMC; Circles: 50mg/kg MPE; Triangles: 100mg/kg MPE; Stars: 150mg/kg MPE and Diamonds: 200mg/kg MPE
Mean wound healing time
The steady contraction of wound with time leads to complete contraction and healing of the wound. Regular monitoring of wound was carried out visually and the day on which wounds healed completely was recorded. The regular monitoring of excision wound showed a dose dependent reduction in the mean wound healing time (Figure 2). The mean wound healing time for CMC treated group was 24 days which declined with MPE treatment and animals receiving 50mg/kg showed a mean wound healing time of 22 days (Figure 2). The increase in the MPE dose to 100 mg further reduced the mean wound healing time, where it was 21 days. The greatest reduction in mean wound healing time was recorded for 200mg/kg where it was 18 days, a reduction of 6 days when compared to CMC treatment (Figure 2).
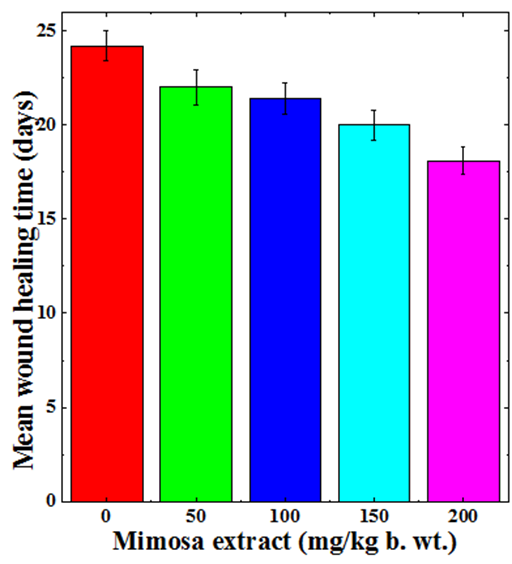
Figure 2 Augmentation in the mean wound healing time of deep dermal excision wound of mice treated with different doses of ethanol extract of Mimosa pudica.
Biochemical profile
The results of various parameters are shown in the Tables 1–2 & Figures 3–10.
Collagen synthesis
The synthesis of collagen increased in a dose dependent manner on both day 7 and 12 post wounding (Figure 3). The lowest but significant (p<0.01) increase was recorded on day 7 post wounding in the mice treated with 50mg/kg b. wt. MPE. With increasing dose, the synthesis of neocollagen also increased and a maximum rise in collagen synthesis was observed for the 200mg/kg b. wt. MPE on day 7 post wounding (Figure 3). The estimation of collagen synthesis on 12 day post wounding showed a decline in the rate of collagen synthesis when compared to day 7 and significant increases were only observed for 150 and 200 mg/kg MPE (Table 1).
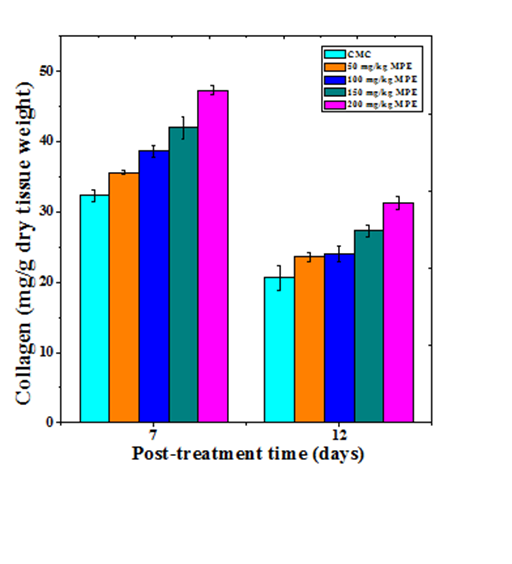
Figure 3 effect of different doses of ethanol extract of Mimosa pudica on the synthesis of collagen at different post wounding days in mice inflicted with deep dermal excision wound.
Hexosamine synthesis
Hexosamine synthesis in the regenerating wound also increased in dose dependent manner on both day 7 and 12 post wounding (Figure 4). The treatment of mice with different doses of MPE caused a significant rise in the hexosamine synthesis on both day 7 and 12 posts wounding (Table 1). The hexosamine synthesis declined with time and the rate of hexosamine synthesis was lower on day 12 when compared to day 7 post wounding (Figure 4). A maximum syhtesis of hexosamine was recorded for 200 mg/kg MPE on both day 7 and 12 post wounding (Figure 4).
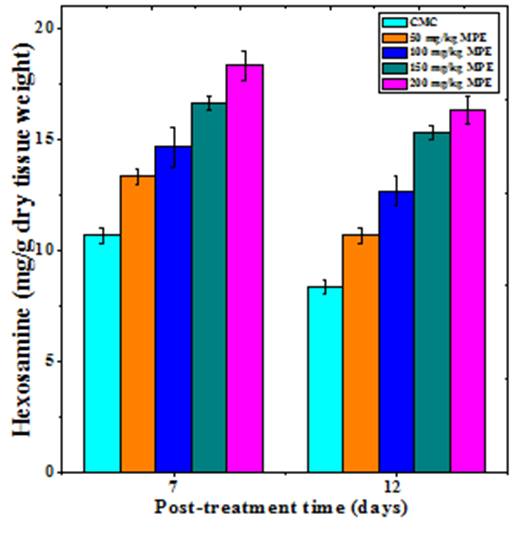
Figure 4 Effect of different doses of ethanol extract of Mimosa pudica on the hexosamine synthesis at different post wounding days in mice inflicted with deep dermal excision wound.
|
MPE |
Mg/g Dry tissue weight ± Standard error of the mean |
|||||||
|
Collagen |
Hexosamine |
DNA |
Nitic oxide |
|||||
|
7 days |
12 days |
7 days |
12 days |
7 days |
12 days |
7 days |
12 days |
|
|
0 (CMC) |
32.33±0.88 |
20.66±1.76 |
10.66±0.33 |
8.33±0.33 |
4.51±0.22 |
3.74±0.08 |
19±1.15 |
18.66±0.66 |
|
50 |
35.66±0.33§ |
23.66±0.66 |
13.33±0.33** |
10.66±0.33** |
4.67±0.5 |
3.97±0.08 |
17.33±0.44 |
16.5±0.5* |
|
100 |
38.66±0.88** |
24.00±1.15 |
14.66±0.88§ |
12.66±0.66** |
5.63±0.46* |
4.88±0.02§* |
16.66±0.88 |
15.5±0.5§ |
|
150 |
42±1.52** |
27.33±0.88§ |
16.66±0.33§* |
15.33±0.33§* |
6.63±0.55§ |
5.89±0.26§* |
13.83±0.16§ |
12.83±0.16** |
|
200 |
47.33±0.66§* |
31.33±0.88** |
18.33±0.66§* |
16.33±0.66§* |
7.2±0.31§* |
6.22±0.15§* |
12.5±0.28** |
11.66±0.16** |
Table 1 Effect of different doses of Mimosa pudica ethanol extract (MPE) on various biochemical parameters in the regenerating wounds of mice at different post wounding days
*= P<0.05; § P<0.01; **= P < 0.001; §*=P<0.0001 and No symbols: Non-significant. When compared to carboxymethylcellulose CMC group (0 mg).
DNA synthesis
The DNA synthesis estimation shows the proliferation status of cells and the DNA synthesis increased in a dose dependent manner at 7 and 12 day post wounding in the regenerating excision wounds of mice treated with different doses of MPE (Figure 5). The lowest doses of 50mg/kg MPE was not very efficient in increasing the DNA synthesis as the increase was statistically non–significant on both 7 and 12 day post wounding (Table 1). However, further increases in MPE dose resulted in a significant rise in the DNA synthesis at both day 7 and 12 post wounding with a maximum elevation for 200 mg/kg MPE (Table 1)).

Figure 5 Effect of different doses of ethanol extract of Mimosa pudica on the DNA synthesis at different post wounding days in mice inflicted with deep dermal excision wound.
Nitric oxide synthesis
The nitric oxide production increased in a dose dependent manner in the regenerating excision wounds of mice receiving different doses of MPE at both 7 and 12 days post wounding (Figure 6). The increase in nitric oxide was non–significant on day 7 post wounding in the mice treated with 50 and 100mg/kg b. wt. of MPE. However a further increase in MPE dose resulted in a significant rise in the NO production of day 7 post wounding (Table 1). The rise in NO production was significantly higher for all MPE doses on day 12 Post wounding (Table 1).
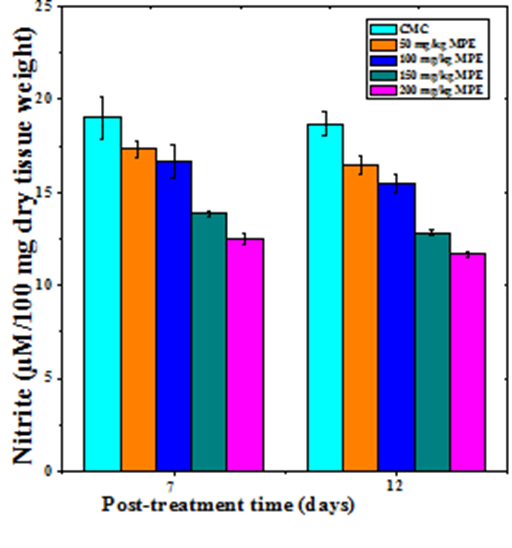
Figure 6 Effect of different doses of ethanol extract of Mimosa pudica on the nitric oxide synthesis at different post wounding days in mice inflicted with deep dermal excision wound.
Estimation of antioxidants
Glutathione
The glutathione concentration in wound granulation tissue of untreated mice was 0.77±0.02 and 1.06±0.05 µmoles/mg of protein at day 7 and day 12 post wounding, respectively (Table 2). The administration of MPE resulted in a time and dose–dependent increase in the GSH concentration in the wound granulation tissue (Figure 7). This increase in GSH concentration was significantly higher than the untreated control (Table 2). A maximum rise in GSH concentration was observed at 7 and 12 days post wounding at a dose of 200 mg/kg b. wt. MPE treatment (Figure7).
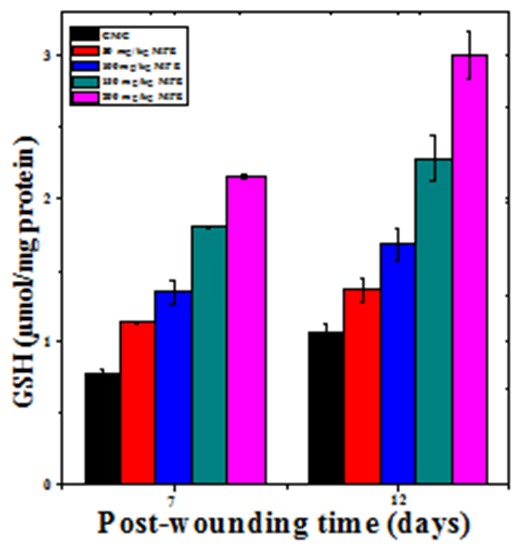
Figure 7 Effect of different doses of ethanol extract of Mimosa pudica on the glutathione contents at different post wounding days in mice inflicted with deep dermal excision wound.
Catalase
The base line catalase activity in wound granulation tissue of untreated mice was 5.70±0.12 and 5.5±0.14 U/mg protein at day 7 and day12 post–wounding, respectively (Table 2). The administration of MPE caused a time and dose–dependent but significant rise in the catalase activity of wound granulation tissue (Figure 8 & Table 2). A maximum rise in catalase activity was observed at 7 and 12 days post–wounding in the granulation tissue of mice receiving 200mg/kg b. wt. MPE treatment (Figure 8).
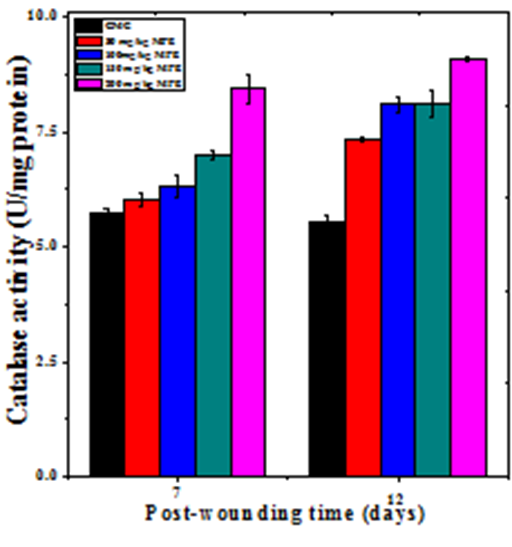
Figure 8 Effect of different doses of ethanol extract of Mimosa pudica on the catalase activity at different post wounding days in mice inflicted with deep dermal excision wound.
|
MPE |
GSH (µmol/mg protein) |
Catalase (U/mg Protein) |
Superoxide Dismutase |
Lipid Peroxidation MDA (nM/mg Protein) |
||||
|
7 days |
12 days |
7 days |
12 days |
7 days |
12 days |
7 days |
12 days |
|
|
CMC (0) |
0.77±0.02 |
1.06±0.05 |
5.70±0.12 |
5.5±0.141 |
13.98±0.93 |
14.22±0.20 |
0.58±0.02 |
0.55±0.01 |
|
50 |
1.12±0.01§* |
1.35±0.08** |
6.02±0.12§ |
7.32±0.04§* |
17.70±0.36§* |
18.96±0.42§* |
0.52±0.01* |
0.50±0.01* |
|
100 |
1.34±0.08§* |
1.68±0.11** |
6.31±0.22§ |
8.08±0.16§* |
21.20±0.55§* |
21.73±0.96§* |
0.48±0.01§ |
0.45±0.01§ |
|
150 |
1.80±0.01§* |
2.28±0.15§* |
6.99±0.09§* |
8.10±0.30§* |
34.83±0.88§* |
35.88±0.74§* |
0.46±0.01** |
0.42±0.01** |
|
200 |
2.15±0.01§* |
2.99±0.16§* |
8.42±0.30§* |
9.08±0.05§* |
42.95±0.45§* |
43.42±0.43§* |
0.43±0.01§ |
0.39±0.01§* |
Table 2 Effect of different doses of 'Mimosa pudica' ethanol extract on various antioxidants in the regenerating wounds of mice at different post wounding days
*= P<0.05; § P<0.01; **= P < 0.001; §*=P<0.0001 and No symbols: Non-significant. When compared to carboxymethylcellulose CMC group (0 mg).
Superoxide dismutase
The base line SOD activity in the regenerating wound granulation tissue of untreated mice was 13.98±0.93 and 14.22±0.2 U/mg protein at day 7 and day12 post wounding, respectively (Table 2). The administration of MPE before wound creation resulted in a time and dose–dependent increase in the SOD activity in the wound granulation tissue (Figure 9). This increase in SOD concentration was significantly higher than untreated control (Table 2). The treatment of mice with 200mg/kg MPE before wounding showed a greatest rise in SOD activity at both 7 and 12 days post wounding (Figure 9).
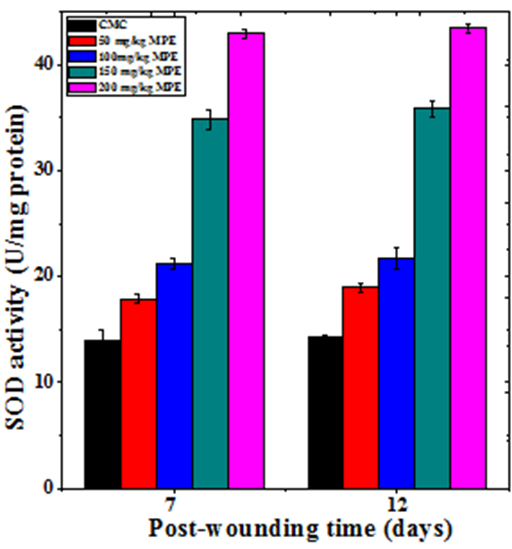
Figure 9 Effect of different doses of ethanol extract of Mimosa pudica on the superoxide dismutase activity at different post wounding days in mice inflicted with deep dermal excision wound.
Lipid peroxidation
The lipid peroxidation (LOO) in wound granulation tissue of regenerating wound of untreated mice was 0.58±0.02 and 0.55±0.01 MDA nM/mg protein at day 7 and 12 post–wounding, respectively (Table 2). Treatment of mice with different doses of MPE before infliction of deep dermal excision wound resulted in a dose–dependent but significant decrease in the LOO of wound granulation tissue at both the post wounding times (Figure 10 and Table 2). The LOO was higher on 7 days post wounding than that of day 12 (Figure 10). The MPE administration reduced the LOO greater on 12 days when compared to day 7 post wounding and the decline in LOO was highest in the mice receiving 200mg/kg b.wt. MPE when compared to the other doses (Figure 10).
Plants and herbs have been used to treat numerous ailments in human beings since the advent of human history and several medicinal systems like Ayruveda and Chinese have been using mainly natural products to treat different diseases in humans. These remedies are time tested and used for several generations without untoward side effects. The effectiveness of medicinal plants in human healthcare may be due their ability to synthesize different phytochemicals for their various metabolic needs or otherwise.5 The use of whole extract will contain all these phytochemicals and will provide maximum curability with no untoward side effects.27 The present study was undertaken to explore the wound healing activity of lajwanti i.e. Mimosa pudicain the Swiss albino mice inflicted with deep dermal excision wound.
Wound healing is an intricate process that progresses in a predefined but orderly manner. It involves the cooperation and coordination of various types of cells which are present in the wound bed or recruited from the margins of the wound and the endothelial system to direct the healing. The process of wound healing usually includes three physiological responses that include inflammation, regeneration and wound remodeling. The inflammation is the immediate response after wounding and is essential to recruit other cells that play a crucial role in the healing process.28 The inflammatory phase in initiated by migration of neutrophils, monocytes and macrophages into the wound bed and their presence initiate debridement and secretion of many cytokines required for the proper healing of the wound.9
The main aim of the injured tissue is to heal the wound as early as possible to avoid other detrimental consequences as a result the new tissue is not the exact replica of the original uninjured tissue.29 This restoration of the tissue is due to the concerted action of blood platelets, neutrophils, monocytes/macrophages, endothelial cells fibroblasts, and keratinocytes.9 In normal course the wound would heals itself but the use of medicine may help the tissue by favourably modulating different regenerative responses and subsequently reducing the defects. The administration of different doses of lajwanti ethanol extract increased the wound repair and regeneration in a dose dependent manner and 200mg/kg MPE accelerated the process of wound healing to the maximum extent as indicated by the increased wound contraction and shortest wound healing time. The periodic measurement of the wound area gives a fairly good indication of wound contraction and the repair status of the wound. As the wound contracts the wounded area shrinks with time and the complete closure of the wound is effected.18,27,30–34 The reports regarding the use of ethanol extract of whole lajwanti plant on the healing of deep dermal excision wound in mice are unavailable. However, earlier studies have reported increased reduction in the wound area after topical application of ethanol or chloroform extract of leaves of lajwanti in rats.35,36 Likewise, topical application of chloroform and methanol extracts of lajwanti roots on the excision wound of rats enhanced wound contraction and epithelialization.17 A similar effect has been observed in wound contraction when the mice were administered with ascorbic acid, curcumin, Nigella sativa extract or topically applied with curcumin.27,30–34
The contraction of wound is caused by the concerted action of contractile myofiborblasts, which are generated as a result of migration of fibroblasts from the unaffected dermis and subcutaneous tissue into the center of the wound granulation tissue and acquire microfibrils, especially the contractile actin and are converted into myofibroblasts. These myofibroblasts usually disappears by apoptosis once the wound heals completely. Their persistence may lead to pathologic condition duing wound healing.37–39 The administration of MPE would have triggered the recruitment of more fibroblasts from wound edges and their conversion into myofibrobalsts leading to greater contraction and early healing of wounds in this group when compared to normal untreated control group. This may have subsequently reduced the mean wound healing time in MPE treated group.
The extracellular matrix (ECM) containing fibronectin, integrins, glycosaminoglycans, proteoglycans, thrombospondins, tenascin, vitronectin, and collagens play a major role in maintaining the structural integrity during wound healing.40 They help in cell migration, storage, and delivery of growth factors and various cytokines to the wound bed.41 The collagens are most important and abundant proteins among ECM and their main function is cell adhesion, cell migration, tissue morphogenesis, tissue scaffolding, and tissue repair. These are fibrillar proteins present in the dermis, which provide structural support to the cells and regulate inflammatory reaction.41 Estimation of collagen has shown that MPE increased its synthesis in a dose dependent manner. There are no reports regarding the enhanced synthesis of collagen by lajwanti. However, ascorbic acid, curcumin and Nigella sativa have been shown to increase the synthesis of collagen, hexosamine and DNA in the regenerating excision wounds of mice.18,27,30,31 The enhanced hexosamine synthesis in the regenerating wounds receiving MPE may be responsible for providing higher strength to the wounds. The topical application of L. japonica has been reported to increase collagen and hexosamine syntheses in the regenerating excision wounds of rats earlier.42 The DNA synthesis is linked to cell proliferation and increased DNA synthesis by MPE may have increase the proliferation of different cells like fibroblasts and keratinocytes leading to the early healing of the regenerating wound in the present study.18,27,31–34 Inflammation is an early event in the healing of regenerating wounds and nitric oxide estimation at day 7 and 12 post wounding has shown that MPE administration reduced the inflammatory response on these days indicating the progression of regeneration and early closure of the wound.
The reactive oxygen species (ROS) are required during wound healing, especially during early stages as they stimulate cell signaling, especially the vascular endothelial growth factor (VEGF) in keratinocytes accelerates angiogenesis and early healing.433,44 However, excess of ROS may have adverse impact on wound healing and they shall be neutralized. The MPE has reduced lipid peroxidation a marker of oxidative stress after wounding and also elevated the glutathione concentration, catalase and superoxide dismutase activity. The ascorbic acid, curcumin and Nigella sativa extract have been reported to elevate GSH, and GSHpx and superoxide dismutase in the regenerating excision wounds of mice earlier.18,27,30,45
The exact mechanism of action of lajwanti extract in accelerating the repair and regeneration of wound healing is not clearly understood and the mechanism for increased contraction and reduced mean wound healing time may be several. The MPE has increased the synthesis of collagen, hexosamine and DNA, which are essential components of wound healing. The alleviation of excess free radicals produced in the excision wound by MPE may be another mechanism of enhanced wound healing. This is supported by the increased GSH concentration and activities of catalase and SOD enzymes and reduction in the lipid peroxidation. Wounding activates the expression of NF–κB as early as ½ hour after wounding and it is essential to elicit proinflammatory responses required for cell proliferation in the regenerating wounds.46 The presence of MPE before wounding may have activated NF–κB immediately after wounding however; it may not have allowed its persistence activation, which could have detrimental effect on healing of wounds. The MPE may have also triggered the synthesis of, PDGF, TGF–β, VEGF and other cytokines that are essential for wound healing leading to the early regeneration and repair of the wounds. The increase level of antioxidants suggests that MPE may have stimulated Nrf2 signaling as a late event leading to early repair of the excision wounds as the activation of Nrf2 signaling has been reported to reduce excess inflammation during repair and regeneration of the wound.47 The increase in wound healing activity may due to the presence of flavonoids including quercetin, leuteolin, and rutin and other phytochemicals like mimosine in the MPE.12
The present study demonstrates that MPE increased the wound contraction in a dose dependent manner and at the same time reduced the mean wound healing time. This acceleration in woundPlants and herbs have been contraction may be due to increased collagen, hexosamine and DNA syntheses and reduced NO synthesis. The other mechanism may be increased antioxidant status at later times that wound have increased the cell proliferation and effected early wound healing. It is also possible that MPE may have stimulated molecular mechanisms where it may have activated the NF–κb, TGF–β, PDGF, VGEF and other cytokines as an early event. However it may have also activated Nrf2 as late event to subdue the inflammatory reactions, which are not required during late stages of wound healing.
This work was carried out under a Grant from University Grants, Government of India, New Delhi to Prof. GCJ.
The authors declare that there are no conflicts of interest.

©2017 Jagetia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.