International Journal of
eISSN: 2381-1803


Research Article Volume 8 Issue 5
Department of Botany, North-Eastern Hill University, India
Correspondence: Diana Kharkongor, Algal Ecology Laboratory, Centre for Advance studies in Botany, Department of Botany, School of Life Sciences, North-Eastern Hill University, Shillong- 793022, Meghalaya, India
Received: May 05, 2017 | Published: September 1, 2017
Citation: Kharkongor D, Ramanujam P (2017) Antioxidant Activities of Four Dominant Species of Trentepohlia (Trentepohliales, Chlorophyta). Int J Complement Alt Med 8(5): 00270. DOI: 10.15406/ijcam.2017.08.00270
The evaluation of antioxidant activities of four Trentepohlia species (i.e. T. abietina, T. arborum, T. diffracta and T. umbrina) in vitro revealed a remarkable result where all four studied species were found to be rich in phenolic compounds. Comparatively T. abietina extract contained the highest amount of total phenolic compound and flavonoids content as well, and itexhibited the maximum scavenging activity with the lowest IC50. The scavenging activity of the four Trentepohlia species extracts was observed to decrease in the following order T. abietina >T. arborum>T. diffracta>T. umbrina. Moreover, both DPPH radical scavenging activity and superoxide anion radical scavenging activity varied significantly between the four species. In case of reducing power, extracts of all four species were concentration dependent where high reducing power was observed at higher concentration and gradually decreased with decrease in concentration of the extracts. Comparing amongst the species, T. abietina again showed highest reducing power at all the concentrations. The result of correlation showed a positive increase in scavenging activities with increase in flavonoids content. This clearly indicated that in this case, the scavenging effect exhibited by the extract of all four species of Trentepohlia mainly the DPPH scavenging activity could be attributed to the presence of mainly flavonoids constituent.
Keywords: trentepohlia, subaerial algae, antioxidant, phenolic, flavonoids, free radicals, dpph, superoxide anions, carotenoids, inhibition concentration
ROS, reactive oxygen species; OH, hydroxyl; H2O2, hydrogen peroxide; TBHQ, tertiary butylhydroquinone; PG, propyl gallate; NBT, nitroblue tetrazolium; DPPH, 2,2-diphenyl-1-picrylhydrazyl; EDTA, ethylenediaminetetraacetic acid; PMS, phenazine methosulphate; ANOVA, one-way analysis of variance
An antioxidant is a substance that is present at low concentrations and significantly delays or prevents oxidation of the oxidizable substrate. Antioxidants are effective because they can donate their own electrons to Reactive Oxygen Species (ROS) and thereby neutralizing the adverse effects of the latter.1 Reactive oxygen species (ROS) is a collective term used for a group of oxidants, superoxide (O2-) radicals, hydroxyl (OH) radicals and hydrogen peroxide (H2O2), which is either free radical or molecular species capable of generating free radicals.2 ROS are generated as by-product of biological reactions such as the mitochondrial respiratory chain or from exogenous factors or environmental stresses.3,4 They are highly reactive transient chemical species formed in all tissues during normal aerobic cellular metabolism, with the potential to initiate damage to the various intracellular components (nucleic acids, lipids, proteins) on which the functioning of normal cell depends.
The most active dietary antioxidants belong to the family of phenolic and polyphenolic compounds. Flavonoids and phenolic acids are the most important groups of secondary metabolites and bioactive compounds in plants and are good sources of natural antioxidants in human diets.5 They are also a kind of natural product capable of scavenging free superoxide radicals, reducing the risk of cancer and protecting biological systems against the harmful oxidative processes on carbohydrates, proteins, lipids and DNA.6-10The most commonly used antioxidants are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT),tertiary butylhydroquinone (TBHQ), and propyl gallate (PG), however, there has been growing concern over their safety and toxicity. Therefore, the development and utilization of more effective antioxidants of natural origin are desired. Of various kinds of natural antioxidants, phenolic compounds have received much attention.11,12 Phenolic antioxidants are reported to quench oxygen-derived free radicals as well as the substrate-derived free radicals by donating a hydrogen atom or an electron to the free radical. The antioxidant activity of phenolics in several systems has been proved to be as active as BHA or BHT.
Algal organisms are rich sources of structurally novel and biologically active metabolites. Many potential bioactive compounds produced by these organisms as primary or secondary metabolites had raised interests in the pharmaceutical industry.13-15 Microalgae are known as rich source of natural antioxidants. They may serve as a continuous and reliable source of natural products, including antioxidants, because they can be cultivated in bioreactors on a large scale.16 Recently, many research works have been carried out for screening of various microalgae for production of natural antioxidants.17-21 Moreover, it was reported that carotenoids are well known class of antioxidants from microalgae that play an important role in quenching reactive oxygen species (ROS) generated during photosynthesis, especially singlet oxygen. Several studies have demonstrated that carotenoids contribute significantly to the total antioxidant capacity of microalgae.22,23 Microalgae such a Haematococcus and Dunaliella are already commercially utilised for production of carotenoids as source of antioxidants, for use as additives in food and feed applications, as well as for use in cosmetics and as food supplements.24,25
Trentepohlia a genus of subaerial algae is widespread in nature and found growing on different substrata. This genus is easily recognisable because of their bright orange-red and yellow coloured patches due to accumulation of carotenoids in the filaments. Although Trentepohlia is the most reported genus on subaerial habitat their studies is mostly concentrated on taxonomy and diversity. Presence of various arytenoids in some species of Trentepohlia was documented by various workers.26-30 However little work had been carried out to analyse the other secondary metabolites and antioxidants compounds such as phenolics and to examine the biological activities of these carotenoids rich subaerial genera. So far only Simic et al.31 had demonstrated the antioxidant and antimicrobial activities of the extract of Trentepohlia umbrina andalsosuggested the microalga as a good source of natural antioxidants.
Trentepohlia is a dominant genus of subaerial algae found growing on different substrata in Shillong, Meghalaya. A total number of 10 species had been reported,32 of which four species were dominant. Presence of high quantity of carotenoids was also documented from these four species of Trentepohlia.33 Hence, this present work was carried out to examine specifically the antioxidant activity of the four dominant species and to throw to light the participation of other antioxidant compounds such as phenolics besides the well known carotenoids.
Algal samples
Four dominant species of Trentepohlia were collected from four different substrata in Shillong Meghalaya; viz. Trentepohlia diffracta (Krempelhuber) Hariot from cemented wall, Trentepohlia arborum (C. Agardh) Hariot from rock surface, Trentepohlia umbrina (Kutzing) Bornet from iron electric pole, and Trentepohlia abietina (Flotow) Hansgirg was collected from bark of Eucalyptus species. Identification of collected Trentepohlia species was done using standard monographs and literature.34-39
Chemicals and reagents
Folin-Ciocalteu reagent, Sodium carbonate, Aluminium trichloride, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), Nicotinamide adenine dinucleotide (NADH), Phenazine methosulphate (PMS), Nitroblue tetrazolium (NBT), Potassium ferricyanide, Trichloroacetic acid, Ferric chloride, Ethylenediaminetetraacetic acid (EDTA), Iron-EDTA, Dimethyl sulfoxide, Ammonium acetate, Glacial acetic acid and Ascorbic acid. Chemicals used were of analytical grade.
Standards
Ascorbic acid, Quercetin and Gallic acid were obtained from Sigma Chemical Co. (St. Louis, MO).
Preparation of algal extract
Air-dried algal material (1g) was extracted using methanol at room temperature. The extract was filtered through filter paper (Whatman no.1) and then evaporated to dryness. The dry crude extract was dissolved in methanol and stored at -20 °C until they were used in the tests.
Quantitative analysis of antioxidative compounds
Determination of total phenolic content: Total phenolic content was determined with Folin & Ciocalteau reagent following Slinkard & Singleton.40 using gallic acid as standard phenolic compound. 1ml of the extract (1mg/ml) in a volumetric flask was diluted with 46 ml of distilled water. 1ml of Folin-Ciocalteu reagent was added and mixed thoroughly. After 3 minutes, 3ml of 2% sodium carbonate was added and then the mixture was kept in the dark at room temperature for 2 hours, and the mixture was shaken frequently every now and then. The absorbance of the mixture was then measured at 760nm in a Cary 100 UV-visible spectrophotometer against blank consisting of all the reagents except the algal extract.
A calibration curve of gallic acid was prepared and the results were expressed as mg GAE (gallic acid equivalent)/ mg dry weight of extract.
Determination of total flavonoid content: Total flavonoid content was determined by spectrophotometric method.41 using quercetin as standard. 1ml of a 2 % methanolic AlCl3 solution was mixed with 1ml of 1mg/ ml extract, and its absorbance was determined at 415nm. The mixture was incubated at room temperature for 10 minutes, and the absorbance was measured at 415nm. Negative control, without extract was used as the blank. A calibration curve of Quercetin was prepared and the results were expressed as μg QE (Quercetin Equivalent) / mg dry weight of extract.
Antioxidant activity
DPPH radical scavenging activity
The DPPH radical scavenging activity of the algal extract was measured using the method described by Brand-Williams et al.42 with some modifications. Two-fold dilution of the extract was made to get a concentration of 500, 250, 125, 62.5 and 31.25μg/ml. Diluted solutions of extract (1ml each) were mixed with 2 ml of methanol solution of DPPH radical (0.05 mg/ml). The mixture was shaken vigorously and allowed to stand for 30 minutes at room temperature. Then the absorbance was measured at 517nm against a blank solution that contained 2ml methanol and 1ml algal extract. A solution containing 2ml DPPH and 1ml methanol was used as the control. Ascorbic acid was used as standard.
The ability of extract to scavenge DPPH free radical was calculated using the following equation:
DPPH scavenging effect % =.(A0 - A1)/A0 × 100
Where A0 is the absorbance of the negative control (2ml of methanol solution of DPPH radical + 1 ml of methanol) and A1 is the absorbance of reaction mixture or standard.
The inhibition concentration at 50% inhibition (IC50) was used to measure the radical scavenging activity. A lower IC50 meant better radical scavenging activity.
Reducing power: The reducing power was determined according to the method of Oyaizu.43 Two-fold dilution of the extract was made to get a concentration of 500, 250, 125, 62.5 and 31.25μg/ml. Each extract (1ml) was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1%). The mixtures were incubated at 50 °C for 20 minutes. Trichloroacetic acid (10%, 2.5 ml) was added to the mixture and centrifuged. Finally, the upper layer (2.5 ml) was mixed with distilled water (2.5 ml) and ferric chloride (0.5 ml; 0.1%). The absorbance of the solution was taken at 700nm in spectrophotometer. Blank was prepared with all the reaction agents without extract. Increased absorbance of the reaction mixture specified that the reducing power is high. Ascorbic acid was used as positive control.
Superoxide anion radical scavenging activity: Superoxide anion radical scavenging activity of the algal extract was measured following Nishimiki et al.44 Two-fold dilution of the extract was made to get a concentration of 500, 250, 125, 62.5 and 31.25μg/ml. Each extract (0.1 ml) was mixed with 1 ml nitrobluetetrazolium (NBT) solution (156μM in 0.1 M phosphate buffer, pH 7.4) and 1 ml nicotinamide adenine dinucleotide (NADH) solution (468 μM in 0.1 M phosphate buffer, pH 7.4). The reaction was started by adding 100μL of phenazinemethosulphate (PMS) solution (60μM in 0.1 M phosphate buffer, pH 7.4). The mixture was then incubated at room temperature for 5 minutes, and the absorbance was measured at 560nm. Phosphate buffer was used as the blank. Decreased absorbance indicated increased superoxide anion radical scavenging activity. Ascorbic acid was used as standard. The percentage inhibition of superoxide anion generation was calculated using the following formula:
Superoxide anion scavenging activity (%) =.(A0 - A1)/A0 × 100
Where A0 is the absorbance of the negative control (All the reacting agents except the extract) and A1 is the absorbance of reaction mixture or standard.
The inhibition concentration at 50% inhibition (IC50) was the parameter used to compare the radical scavenging activity. A lower IC50 meant better radical scavenging activity.
Statistical analysis
Assays were performed in triplicate and results are shown as mean ± standard deviation. Calculation of linear correlation coefficient and Pearson correlation analysis were carried out using MS Office Excel 2007 and XLSTAT 2009 respectively. One-way analysis of variance (ANOVA) was used to find out the significant difference between the samples. A statistical significance of p < 0.05 was considered to be significant.
Total phenolic and flavonoid content
Total phenolic and flavonoid content of methanol extract of Trentepohlia abietina, T. arborum, T. diffracta and T. umbrina are presented in Table 1. T. abietina contained the highest amount of Phenolic compound (75.69 ± 1.8 μg GAE/mg of dry weight of extract) followed by T. arborum (28.75 ± 2.9 μg GAE/mg of dry weight of extract) and T. umbrina (27.55 ± 2.2 μg GAE/mg dry weight of extract) and least was in Trentepohlia diffracta (24.87 ± 1.2 μg GAE/mg dry weight of extract). The flavonoid constituent of the extract was highest in T. abietina (25.32 ± 1.2 μg QE/mg of extract) and followed by T. arborum (22.82 ± 3.4) and T. diffracta (20.21 ± 0.8) and lowest amount was observed in T. umbrina (17.34 ± 1.6 μg QE/mg of extract). The phenolic and flavonoid content varied significantly between the four species (P= 0.000004 and P= 0.0002 respectively).
|
Phenolics content |
Flavonoid content |
|
|
Trentepohlia abietina |
75.69 ± 1.8 |
25.32 ± 1.2 |
|
Trentepohlia arborum |
28.75 ± 2.9 |
22.82 ± 3.4 |
|
Trentepohlia diffracta |
24.87 ± 1.2 |
20.21 ± 0.8 |
|
Trentepohlia umbrina |
27.55 ± 2.2 |
17.34 ± 1.6 |
Table 1 Total phenolics and flavonoid content in methanol extract of Trentepohlia abietina, T. arborum, T. diffracta and T. Umbrina
DPPH radical and superoxide anion scavenging activity
DPPH radical scavenging and superoxide anion radical scavenging activity of four species of Trentepohlia extract are summarised in Table 2. DPPH scavenging activity and superoxide anion scavenging activity of methanol extract of four species of Trentepohlia at different concentrationvaried between the species (Figures 1 & 2).Amongst the four species T. abietina exhibited the maximum scavenging activity with the lowest IC50 value (197.93 ± 1.7μg/ml), followed by T. arborum (387.25 ± 1.3 μg/ml), T. diffracta (484.71 ± 0.9 μg/ml) and T. umbrina (598.77 ± 2.0 μg/ml).
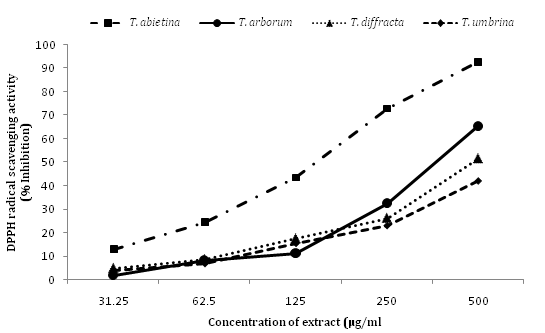
Figure 1 DPPH radical scavenging activity expressed as percentage inhibition of the four Trentepohlia species extract at different concentration.
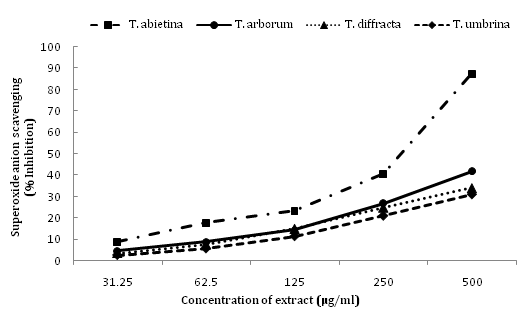
Figure 2 Superoxide anion scavenging activity expressed as percentage inhibition of the four Trentepohlia species extract at different concentration.
|
DPPH radical scavenging activity |
Superoxide anion scavenging Activity |
|
|
Trentepohlia abietina |
197.93±1.7 |
281.68±1.9 |
|
Trentepohlia arborum |
387.25±1.3 |
588.58±1.6 |
|
Trentepohlia diffracta |
484.71±0.9 |
712.82±1.1 |
|
Trentepohlia umbrina |
598.77±1.8 |
801.12±0.7 |
|
Reference standard |
||
|
Ascorbic acid |
7.40±0.1 |
117.69±0.2 |
Table 2 DPPH radical scavenging activity and superoxide anion scavenging activity of methanol extract of Trentepohlia abietina, T. arborum, T. diffracta and T. Umbrina
Similarly, in case of superoxide anion scavenging activity, T. abietina showed better scavenging capacity than the other species of Trentepohlia with lowest IC50 value (281.68 ± 1.9μg/ml). T. umbrina in both the cases showed lesser scavenging activity with higher IC50 value of 598.77 ± 1.8 and 801.12 ± 0.7 respectively.
The scavenging activity of the extracts of four Trentepohlia species decreased in the following order T. abietina >T. arborum>T. diffracta>T. umbrina. However, comparing with Ascorbic acid as reference standard, Trentepohlia extracts showed lower DPPH radical and superoxide anion radical scavenging activity. A significant difference could be observed in both DPPH radical scavenging activity (P= 0.000000007) and superoxide anion radical scavenging activity (P= 0.0000001) between the four species of Trentepohlia.
The correlation between phenolic content and flavonoids content with DPPH radical scavenging activity (Figure 3) and with Superoxide anions (Figure 4) of the four extract taken in term of IC50 was further carried out. The result showed no correlation at all between phenolic content with either DPPH radical and superoxide anion scavenging activity, however, a strong correlation was observed only between flavonoids content with DPPH scavenging activity of the extract (p=0.01, R² = 0.96), whereby an increased in flavonoids content lead to a lower IC50 values.
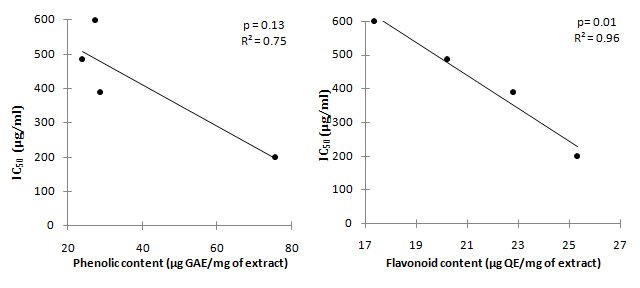
Figure 3 Correlation between DPPH radical scavenging activity IC50 (μg/ml) with Total phenolic content and flavonoid content.
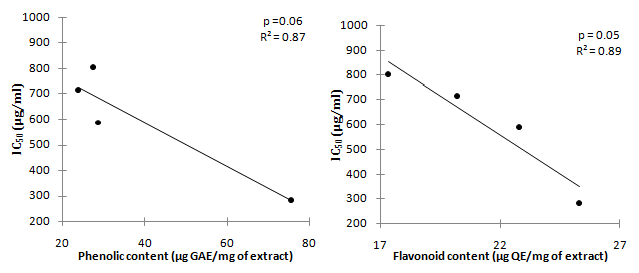
Figure 4 Correlation between superoxide anion scavenging activity IC50 (μg/ml) with Total phenolic content and flavonoid content.
Reducing power
The reducing power in all the four species of Trentepohlia extract was concentration dependent. High reducing power was observed at higher concentration and gradually decreased with decrease in concentration of the extracts. The absorbance of Trentepohlia extract is presented in Table 3. Comparing with Ascorbic acid as reference standard, four species of Trentepohlia exhibited moderate reducing power.
|
1000 µg/ml |
500 µg/ml |
250 µg/ml |
125 µg/ml |
62.5 µg/ml |
31.25 µg/ml |
|
|
Trentepohlia abietina |
0.55 |
0.38 |
0.29 |
0.24 |
0.20 |
0.17 |
|
Trentepohlia arborum |
0.55 |
0.36 |
0.27 |
0. 24 |
0.18 |
0.18 |
|
Trentepohlia diffracta |
0.34 |
0.29 |
0.25 |
0.19 |
0.17 |
0.15 |
|
Trentepohlia umbrina |
0.18 |
0.14 |
0.09 |
0.05 |
0.02 |
0.01 |
|
Reference Standard |
||||||
|
Ascorbic acid |
1.98 |
1.96 |
1.92 |
1.74 |
0.82 |
0.58 |
Table 3 Reducing power of methanol extract of Trentepohlia abietina, T. arborum, T. diffracta and T. Umbrina
Comparing amongst the species, Trentepohlia abietina showed highest reducing power at all the concentrations followed by Trentepohlia arborum, T. diffracta and it was minimum in T. umbrina. The reducing power between the four species of Trentepohlia varied significantly (P=0.000001) at different concentration (P = 0.03).
The present evaluation of antioxidant activities of four Trentepohlia species in vitro revealed a remarkable result. All four species of Trentepohlia studied were found to be rich in phenolic compound. Comparatively T. abietina extract contained the highest amount of total phenolic compound and flavonoids content and also exhibited the highest DPPH radical scavenging activity, superoxide anion radical scavenging activity and reducing power. An increased in phenolic and flavonoids content was observed to be accompanied by higher scavenging activities (low IC50 values) which indicated that phenolics might be the constituents that are also responsible for the scavenging effect in these four Trentepohlia species. However taken into account the correlation result, it clearly indicated that total phenolic content itself in this case did not influence the scavenging activities of the Trentepohlia extract. Although some reports had shown the correlations between antioxidative activities of algae and phenolic content,45,46 this might not always be true as total phenolic content in many cases did not correlate with antioxidative activities as reported by other researchers.47 Hence in this present scenario the scavenging effect exhibited by the extract of four species of Trentepohlia mainly the DPPH scavenging activity could be attributed to the presence of mainly flavonoids constituent. Therefore with increase in flavonoid content of the Trentepohlia extract there was a significant increase in scavenging activities. Hence T. abietina with significant higher amount of flavonoids showed better scavenging properties followed by T. arborum, T. diffracta and T. umbrina respectively. It was also well documented that Flavonoids are a class of secondary plant phenolics with significant antioxidant and chelating properties. Several workers had also reported that Flavonoids can directly scavenge hypochlorous acid, hydroxyl, singlet oxygen and lipid peroxyl radicals, by metal chelation and by inhibiting lipoxygenase activity.48 Rice-Evans et al.49 explained that the antioxidant properties of flavonoids and other phenolics are mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators and singlet oxygen quenchers. Simic et al.31 reported superoxide radical scavenging activity of methanol extract in T. umbrina based on its ability to destroy the Superoxide radical generated from the PMS-NADH reaction.
Since presence of high quantity of carotenoids in these four species of Trentepohlia had also been reported,33 the antioxidant activities contributed by carotenoids besides flavonoids must not be ruled out in this case. Martinez & Barbosa.50 reported the ability of carotenoids to deactivate singlet oxygen by physical quenching. According to them carotenoids could quench radicals by hydrogen atom transfer or by accepting electrons from radicals. Role of carotenoids as antioxidants had also been widely published in many microalgae especially Dunaliella salina and Haematococcus pluvialis in the recent years.51-56 Therefore, in this situation too Carotenoids also could contribute to the increased scavenging activities of Trentepohlia extract along with flavonoids.
Assessment of antioxidant activities of four dominant species of Trentepohlia indicated that the extract from all four species had considerable antioxidant potential in vitro. All the four studied species contained considerable amount of flavonoids contents which are potent antioxidants as shown by their high scavenging capacity and reducing activity. From this work it can also be concluded that flavonoids which are potent antioxidant compounds contributed to the antioxidant properties of four Trentepohlia species besides the well documented carotenoids. Exhibition of good antioxidant activities by these four species (T. abietina, T, arborum, T. diffracta and T. umbrina) also added to the knowledge on potential of Trentepohlia as a candidate for natural source of antioxidants. Hence, further researches in this line, especially developing fast culturing methods are very essential to make use of this natural resource commercially.
We are thankful to North Eastern Hill University for the financial support and to the Head of Botany Department, for providing us all the laboratory facilities.
This work had been done by us in the Algal Ecology Laboratory in North-Eastern Hill University, a Central University of India.
Therefore, “we the authors hereby declare that there is no conflict of interests regarding the publication of this article”.
“We also declare that this manuscript has not been published elsewhere and is not currently under consideration by another journal. The submission of the Manuscript is approved by the Institution.
None.

©2017 Kharkongor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.