International Journal of
eISSN: 2574-9862


Research Article Volume 8 Issue 2
1Department of Nutrition and Animal Husbandry, University of Veterinary Medicine and Pharmacy, Košice, Slovakia
2Department of Public Veterinary Medicine and Animal Welfare, University of Veterinary Medicine and Pharmacy, Košice, Slovakia
3Department of Husbandry and Development of Animal Health, Faculty of Veterinary Medicine, Menoufia University, Shebin Alkom, Menoufia, Egypt
4Department of Pathobiochemistry, Faculty of Pharmacy, Meijo University Yagotoyama, Tempaku-ku, Nagoya-shi, Japan
Correspondence: František Zigo, University of Veterinary Medicine and Pharmacy in Košice, Department of Nutrition and Animal Husbandry, Košice, Komenského 73, 040 01, Slovakia, Tel +421 908689722
Received: May 25, 2024 | Published: June 5, 2024
Citation: Kalinaj B, Zigo F, Farkašová Z, et al. The influence of health status on the changes of gut microbiota and performance of racing pigeons. Int J Avian & Wildlife Biol. 2024;8(2):49-53. DOI: 10.15406/ijawb.2024.08.00213
During the race season of racing pigeons, the demands on their care, zoohygienic conditions, balanced feeding and maintaining an optimal state of health increase. Any deviation in the mentioned factors causes a stressful situation associated with the changes in the bacterial representation in the digestive tract, weakens the individual´s state of health and thus reduces his performance. The work aimed to monitor the health status of racing pigeons and evaluate their performance during one racing season. From 60 pigeons, swabs from the cloaca and crop were taken at regular intervals at the beginning, in the middle and at the end of the race season with a focus on monitoring the representation of the intestinal microflora and the most common diseases. The pigeons were also clinically examined with subsequent sampling for monitoring coccidiosis, trichomonosis and respiratory syndrom during the individual phases of the race season. The total count of bacteria (TCB) in both the cloaca and the crop did not show any significant variation in their abundance throughout the entire racing season. On the other hand, with increasing stress and the occurrence of diseases, the coliform bacteria (CB) in the cloaca and crop was increased in the end of the racing season. Of the identified bacteria Ent. columbae, E. coli, Ent. faecium, Ent. gallinarum a S. intermedius were most frequently represented in swabs from the cloaca. As in the cloaca, the swabs from the crop were most frequently represented by Ent. columbae a E. coli together with lactobacilli and Ent. gallinarum. At the end of race season, E. coli, Ent. faecium, Ent. columbae and staphylococci were significantly represented in the cloaca and in the crop Ent. columbae, E. coli, Ent. gallinarum together with staphylococci again. By comparing the prevalence of Eimeria spp. and Trichomonas spp. before, during and at the end of the race season were noticed their increased occurrence at the end of the season. From the race placements, we found that in pigeons in which the prevalence of opportunistic pathogens Staphylococcus spp. a Ent. gallinarum from swabs of the cloaca and crop at the lowest level together with the occurrence of Eimeria spp., they achieved constant results the entire race season and also achieved the best points position.
Keywords: racing pigeons, stress, microflora, Ent. columbae, E. coli, Eimeria spp
Breeding of racing pigeons is one of the hobbies with admiration for the flight of pigeons with an emphasis on the development of their orientation and performance. The race season is very difficult for pigeons from several aspects. It is mainly in terms of health, fitness and orientation. In addition to these aspects, pigeons also come into contact with other pigeons mainly during basketing or transport and are exposed to various infectious agents.1,2
Breeders demand better and better performance from their individuals, in which they are exposed to enormous pressure due to the short time for regeneration. Only by perfectly managing stressful situations, the number of which only increases, is it possible to achieve high-quality performance. Furthermore, it is necessary to keep the pigeons in the best possible state of health. If pigeons are to perform well, they need proper condition, to ensure these factors, it is necessary to provide the pigeons with a balanced feed that contains all the necessary energy components, minerals and vitamins.3 Attention should also be paid to the zoo technical equipment of the pigeon’s lofts and the use of an effective prophylactic program. The quality of nutrition, the equipment of the lofts, the effect of the prophylactic program and the influence of stress factors directly reflect the representation of the bacterial microflora in the digestive tract of pigeons and the occurrence of diseases in the breeding.2 Any deviation in the mentioned factors causes stressful situation and it associated with a change in bacterial representation in the digestive tract, weakens the health of the individuals, which is reflected in the reduced performance of the competitors.4 Due to the different level of training and racing load of pigeons during the flight season, the aim of the work was to monitor the development of the bacterial microflora at the beginning, during and at the end of the racing season with the occurrence of the most common diseases and their impact on the performance of the racers.
Health assessment and sampling
In the practical part of the work, 60 racing pigeons of both sexes from private breeding with registration in the Basic Organization of Racing Pigeons Breeding in Spišské Podhradie, Slovakia, were included. The racing season consisted of 19 races held from 3rd of May to 31st of July 2022. The pigeons were released from short distances (<150 km) to long distances (<800 km) under constant weather conditions between 5.30 and 6.30 am. Examination of the flock was carried out at the beginning, in the middle and at the end of the racing season during training, feeding and rest. The monitoring of racing pigeons ensured comprehensive evaluation of individuals both in motion and during rest. Individual deviations of the pigeons from their physiological state were monitored. The clinical examination of individual body parts consisted of an inspection of the beak cavity, eyes, wings and cloaca. Examination of the musculature and flexibility of the wings consisted of thorough palpation according to Aiello et al.5
The first clinical examination with the collection of swabs from the cloaca and oropharynx according to our previous study Zigo a kol.6 was carried out before the start of the racing season, where 60 pigeons were examined, supplemented by a coprological examination for the determination of Eimeria spp. according to Balicka-Ramisz a Pilarczyk,7 taking samples from the beak cavity and crop for the determination of trichomoniasis8 and the respiratory complex according to Rupiper a Ehrenberg.9 During the second examination, in the middle of the race season, 51 pigeons were examined due to the loss of some individuals. At the end of the race season, 53 pigeons were examined due to the return of two pigeons from the previous race.
Diagnostic and laboratory analysis
After swabs collection from the cloaca and oropharynx, the samples were placed in a tube with physiological solution and transferred to the laboratory. During 24 hours at a temperature of 37°C, the inoculum from the test tubes was applied to culture media such as meat peptone agar (MPA) and Endo agar (EA) for determination of TCB and CB. At the same time, swab samples were inoculated onto blood agar with the addition of 5% defibrinated ram's blood and selective agar (Bair Parker agar, Muller Hilton agar – Oxoid, UK) under aerobic conditions. Using Enterotest 24 and Enterotest 16 (Erba, Lachema, Czech Republic), fermentative gram-negative bacteria from the Enterobacteriaceae family were determined by standard biochemical procedures. Staphytest 24 and Streptotest 24 (Erba, Lachema, CR) were used to identify staphylococci and streptococci.6
For the detection of lactobacilli were swabs of the cloaca and oropharynx put into the acidified MRS broth and after inoculated into MRS medium (BTL, Poland) supplemented with 0.05% (w/v) Cysteine hydrochloride (Sigma-Aldrich, Poland) (MRS-cys) incubated at 37°C for 24 h and 48 h in 5% CO2. Typical colonies grown on MRS medium and only catalase-negative Gram-positive rods were considered as presumptively belonging to the genus Lactobacillus and identified using API kit (API 50 CHL, bioMėrieux, France) by software APILAB Plus (Ver. 3.3.3; bioMėrieux, France).10
Statistical analysis
Chi quadrate test (χ2 test) was performed for comparison of isolated strains from swabs of the cloaca and oropharynx at the start, before and after flying season with the comparison of most common diseases and performance. The total count of bacteria and coliform bacteria were converted to decimal logarithmic values (Log10 CFU/ml) and submitted for an Analysis of Variance (ANOVA). The statistical differences were considered as significant at the level of 0.05 or less.
The basis for successfully managing the race season is maintaining of good state of health and managing all prophylactic measures during the season.11 By monitoring a selected group of racing pigeons at the beginning, during and at the end of the race season were obtained changes of the representation of intestinal microflora and health status with an impact on their performance. The representation of microorganisms in the digestive tract can also be called „pigeon house microflora“. This microflora, from the point of view of the production of various substances and digestive enzymes in the body of the pigeon, helps to digest to receive feed and to maintain a stable pH. However, during the overgrowth or colonization of the intestine by the same bacteria, but with the different spieces name, which may not cause infection in neighboring flocks, serious problem can occur that can seriously affect the health of the pigeon or the entire flock. This effect mainly occurs during stressful events or after contact with other pigeons during transport in one basket. It follows, that the race season, which conditions these effects for us, is the main link with which the change in the representation in the microorganisms of the digestive tract is connected.4
In the monitored pigeons, the high TCB was determined from cloaca swabs, which indicates natural colonization of the gastrointestinal tract. The TCB levels in both the cloaca and the crop did not show any significant variation in their abundance throughout the entire racing season. On the other hand, with increasing stress and the occurrence of diseases, the CB in the cloaca and crop was increased in the middle and at the end of the racing season (Graph 1). Of the identified bacteria a relatively high representation of Ent. columbae was recorded at the beginning of the race season, namely 78,3% in the cloaca. Isolates of E. coli and Ent. faecium had also a high representation from cloaca swabs (Graph 2).
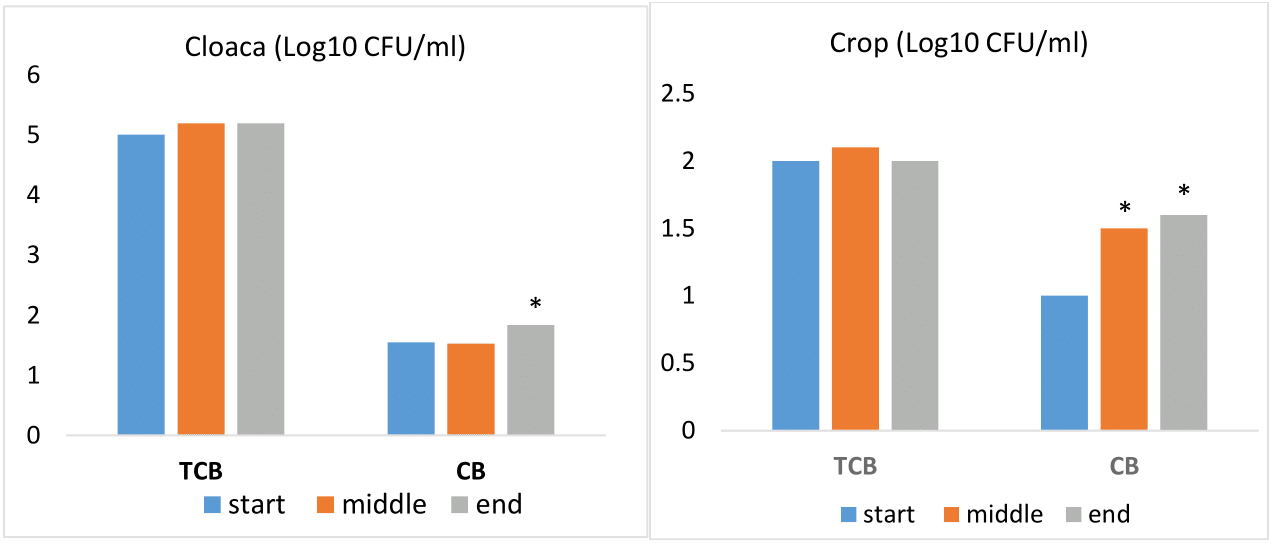
Graph 1 Comparison of TCB and CB in the cloaca and crop at the beginning, during and at the end of the racing season.
Note: TCB; total count of bacteria, CB; coliform bacteria, *statistical significance (p < 0.05).
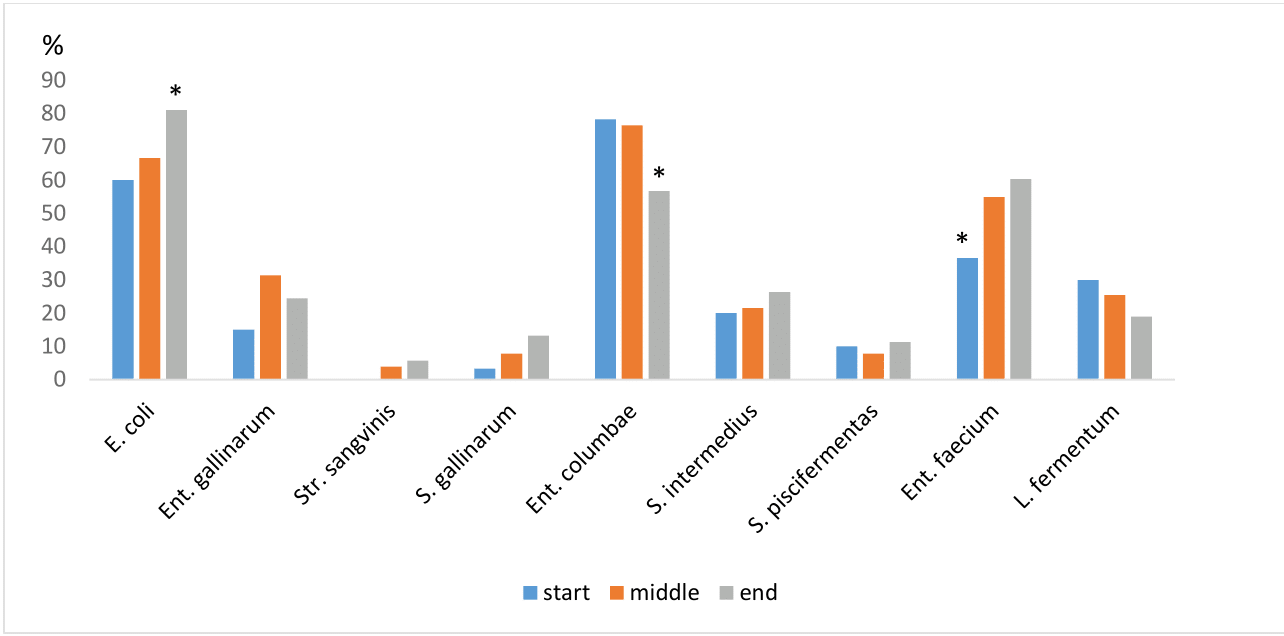
Graph 2 Comparison of bacteria in the cloaca at the beginning, during and at the end of the racing season.
Note: *statistical significance (p < 0.05).
Devriese12 has confirmed with his study that Enterococcus columbae is a typical commensal of the digestive tract of pigeons this bacteria is species-specific for pigeons and is not isolated from any other animal species. This statement is consistent with the results of our study, when the occurrence of Ent. columbae in 39 of 51 samples represented 76,5% prevalence and in the third cloaca collection at the end of the in 30 of 53 samples, demonstrating 56%. Gradually reduced incidence of Ent. columbae was recorded in the middle and at the end of the season, which may be consequence of the increasing incidence of Eimeria spp. with the onset of immunosuppression of the organism due to exhaustion and short period of regeneration.
In the study Guenther13 compared the bacterial microflora in the digestive tracts between raptors and grain-eating birds including pigeons. The author recorded the occurrence of E. coli, Enterobacter spp., Klebsiella spp. a Erwinia spp., in the intestine of birds. In particular, an increased number of positive samples for the presence of staphylococci and streptococci in the intestine of pigeons, Weir14 describes that the majority of individuals with clinical signs of coccidiosis or endoparasitosis had a higher prevalence of E. coli in the digestive tract than the enterococci. The higher occurrence of E. coli during the racing season can also be influenced by incorrect and poor-quality nutrition, or extreme strain on the body with short regeneration between individual races. E. coli infection (colibacillosis) manifests itself with similar clinical symptoms as infection caused by Salmonella spp., which may also be relevant in our study, where E. coli was diagnosed in most individuals in cloacal swabs as well as from the crop. Many time is problem with interpretation findings of the presence E. coli and pigeons. This is because E. coli normally inhabits the intestinal tract of all animals and birds. There are a number of different strains and many are species-specific. Bacteria E. coli can be found in most normal and healthy pigeons without symptoms of another clinical disease which was confirmed from previous studies.3,4 If E. coli is identified in other parts of the body than in the gut, including the colon, then this indicates potential health problem that usually requires treatment. E. coli is sometimes identified in the crop. It is not a normal resident of the crop, which is why it is unusual there. Especially in pigeons in which E. coli was isolated together with staphylococci from the crop they were more susceptible to diseases such coccidiosis and respiratory syndrome.
Some disease causing with pathogenic avian E. coli (APEC) strains can cause disease in even the strongest pigeons. Many strains of APEC in pigeons are simply opportunistic, waiting to cause disease if something stresses the birds. It can be a persistent environmental or breeding problem, or another concurrent opportunistic pathogen.7 An explanation of the fact that the discovery of opportunistic pathogens that do not necessarily cause disease in the digestive tract can be found in the work of Chaucheyras and Durand15 who define the competitive exclusion of 2 competing almost identical species. These bacterial species cannot exist in one space both at the same time. They compete for space, which is eventually colonized by a more dominant flora. An important role in this settlement is also played by the prophylactic program, which, if carried out appropriately in the breeding, becomes a guarantee of obtaining healthy and resistant individuals. They thus have a sufficiently developed commensal microflora, which is strong enough to fight the pathogenic one, thus becoming dominant. Ent. columbae, E. coli was most often represented in the crop swabs during and at the end of the racing season. Their ratio was changing, and that was at the end of the race season with Ent. gallinarum and staphylococci affiliation. According to Sun et al.16 who monitored the representation of the bacterial microflora in birds, including pigeons and raptors, found that Staphylococcus spp., Streptococcus spp. and Enterococcus spp. they are a natural commensal of the crop. This is also confirmed by our study, where Ent. gallinarum occurred during all three samplings, especially in the middle and at the end of the race season (Graph 3). Staphylococci and streptococci were confirmed in our study from swabs of the crop and beak cavity, which can cause infections in various stressful situations. Infections caused by staphylococci or streptococci are not among the primary ones, but rather we encounter them secondarily as associated with other diseases such as mycoplasmosis, ornithosis, etc.
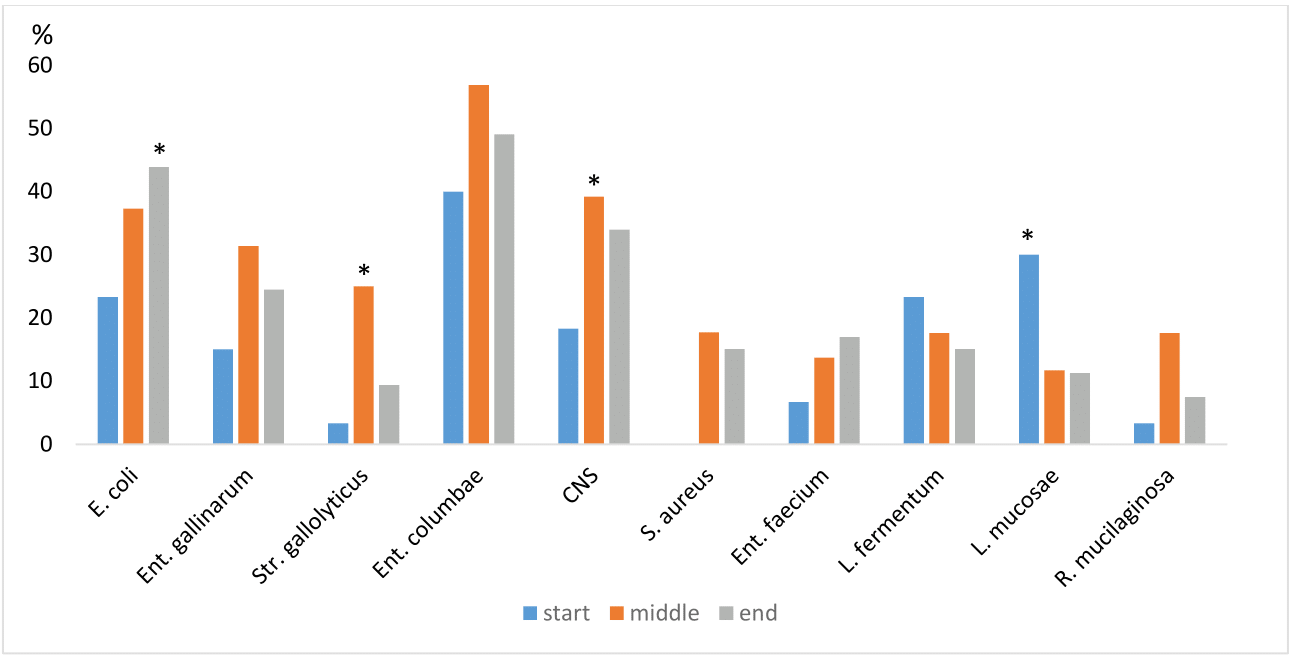
Graph 3 Comparison of crop bacteria at the beginning during and at the end of the race season.
Note: *statistical significance (p < 0.05).
Among the most common clinical symptoms, we observe: skin rashes, reluctance to move, redness around the feather follicles, sneezing and nasal discharge, loss of appetite, depression, fluffy feathers, watery diarrhea and weight loss.17 An increased incidence of staphylococci from crop swabs was recorded in pigeons with respiratory syndrome, especially at the end of the race season. Scullion18 confirmed that the occurrence of coccidiosis in feces may not always be clinically manifested and that adults can overcome this disease. The evaluation of the number of protozoa from the feces samples did not correlate with clinical manifestations, which means that in adults this disease manifests itself as a secondary factor due to the combination of several factors with immunosuppression of the organism. By evaluating the results of our work, we are inclined to this statement, because despite the samples positive for Eimeria spp. with an infection intensity estimate of +, these individuals showed no signs of disease. However, their performance was reduced, which was confirmed by obtaining fewer points compared to healthy individuals (Garph 4). Coprological examination of feces (Eimeria spp.) and from sampling from oropharynx for the diagnosis of Trichomonas spp. we did not confirm their presence in any of the investigated pigeons in the monitored breeding at the beginning of the race season. Their highest incidence was recorded at the end of the season, but all positive individuals were without clinical symptoms (Graph 5). These findings point to a well-set and implemented prophylactic program in monitored pigeon breeding that is effective and helps to cope with frequent infectious agents.
The results of the work show that the bacterial microflora in the digestive tract of pigeons has a variable representation during the race season. Cloaca swabs had a stable representation of Ent. columbae, a E. coli in pigeons with the best point location and the lowest incidence of monitored diseases. In pigeons, which staphylococci and Ent. gallinarum from crop and cloacal swaps showed an increased incidence of coccidiosis and trichomoniasis with poor performance.
Through successive examinations during the race season, we found that pigeons that also maintained a stable microflora achieved the best scoring results and were able to best cope with stressful situations throughout the season. Also through observation and clinical examination during each sampling, we found that these individuals showed the highest well-being and vitality in the loft.
The study was support by grant KEGA no. 011UVLF-4/2024: Improving the quality of practical teaching with the support of animal breeding and higher education for students from the subject of Animal husbandry.
The authors declared that there are no conflicts of interest.

©2024 Kalinaj, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.