International Journal of
eISSN: 2574-9862


Research Article Volume 4 Issue 2
1Department of Animal husbandry, University of Pavol Jozef Šafárik in Košice, Slovakia
2Department of Physiology, University of Pavol Jozef Šafárik in Košice, Slovakia
3Department of Pharmacology, University of Pavol Jozef Šafárik in Košice, Slovakia
4Public Veterinary Medicine and Economics, University of Veterinary Medicine and Pharmacy, Slovakia
Correspondence: František Zigo, University of Veterinary Medicine and Pharmacy, Department of Animal husbandry, Košice, Komenského 73, 040 01, Slovakia, Tel +421-908-689-722
Received: February 19, 2019 | Published: March 15, 2019
Citation: Zigo F, Ondrašovi?ová S, Zigová M, et al. Influence of the flight season on the health status of the carrier pigeons. Int J Avian & Wildlife Biol. 2019;4(2):26-30. DOI: 10.15406/ijawb.2019.04.00148
Carrier pigeons are highly valued for their outstanding orientation skills and high performance that they display during races. The breeding of the carrier pigeon is very important for these reasons. Increased stress and short time for regeneration during the race are an important factors that significantly affects the health of racing pigeons and their composition of bacterial microflora. The aim of this study were to observe the health status and changes in bacterial microflora of 80 racing pigeons in age 1.5 - 4 years originated from holding on west of Slovakia, which for three months completed eighteen races at a distance of 120 - 1150 km. At the beginning and at the end of the race season were taken the samples of swabs from cloaca and oropharynx. Comparing the most common diseases of pigeons, were found an increased incidence of coccidiosis (52.2%), trichomoniasis (31.9%), and respiratory syndrome (7.2%) after the race season. The enterococcal and streptococcal intestinal flora of pigeons during the race season was dominated and composed of mainly host specific bacteria by Ent. faecalis, Ent. faecium, Ent. columbae, E. coli, Str. gallolyticus and Str. faecalis. Results are showing that also despite increased percentage of cultivation opportune pathogens, especially coagulase negative and positive staphylococci and increased prevalence of coccidiosis, trichomoniasis and respiratory syndrome, the pigeons are able to provide flight performance and cope with changes in their organism during this difficult race period.
Keywords: racing pigeons, coccidiosis; trichomoniasis, intestinal flora, enterococci, staphylococci
The breeding of the carrier pigeon is very important for these reasons. Nowadays, each breeder knows that it is not only targeted breeding and associated genetic predisposition of individuals but also a combination of other factors such as feeding, dietary supplements, appropriate zoohygienic conditions, prophylactic plans, adequate training load and taking into account the number of races.1,2 The most vulnerable to infections are racing and highfliers pigeons, as they perform a large number of flights in the so-called racing season. This leads to substantial exhaustion of birds and, consequently, increases their susceptibility to various diseases.3–5 The diseases that affect pigeons are divided in to contagious diseases and non contagious diseases. Viruses, bacteria, fungi and parasites are the main causes for contagious diseases while non-contagious diseases are resulted from the absence of proper nutrients as well as poisonous elements in the food.6
The main infectious illnesses of racing pigeons include: viral diseases, chlamydophilosis and parasitic diseases such as lice, mites, roundworms (Ascaridia, Capillaria), tapeworms, coccidiosis, hexamitosis, trichomonads and trematodes. The bacteria Salmonella typhimurium, Escherichia coli, streptococci, coagulase-positive (CPS) and negative staphylococci (CNS) and parasitic organisms Eimeria columbanum or Eimeria labbeanna and Trichomonas gallinae are the most common causes of clinically symptoms in stressed pigeons, which leads to reduced power in the bird and increases its number of losses.7,8
One of the most common parasitic diseases is coccidiosis (Figure 1). This parasitic protozoon organism affects the bird’s intestines. Two types of coccidia affect pigeons: Eimeria columbanum and Eimeria labbeanna. The clinical disease due to these organisms is identical. Coccidia in the intestines produce ‘oocysts’, (Figure 2) which are passed out in the faeces. These mature in the environment and will then affect other birds if ingested. Most adult birds carry low levels of these parasites. Only when large numbers of parasites are present is treatment necessary. Usually raised levels of coccidial oocysts are associated with sub-optimal performance but in young birds and adults under stress an acute clinical form of the disease may be seen. The parasite affects the lining of the intestine causing diarrhoea and blood may be present. Affected birds are depressed, rapidly become emaciated and may die.9–11
The second most common cause of reduced pigeon performance is the canker (Figure 2). Canker is caused by a parasitic organism called Trichomonas colombi and three forms are recognized that affect the pharynx (throat), navel and internal organs respectively. The majority of adult pigeons are symptomless carriers of the organism but clinical cases may occur if the bird is under stress and in young pigeons the disease may be severe and even fatal. The disease is spread from adults to squabs in the crop milk and between pigeons by the oral route.7,10
Additionally, birds may contract combined infections, known as “ornithosis complex”, "ornithose," "ornithosis complex," "coryza," and "one-eye colds." This group disease can be caused by infection with Chlamydia psittaci, Pasteurella, or mycoplasma organisms. Other gram-negative bacteria E. coli, Yersinia spp., Enterobacter spp., and viral agents such as herpesvirus may also play a role.7,12 The aim of this study was to characterize the most common diseases and changes in bacterial microflora of carrier pigeons during the race season.
Animals and samples
The study included 80 pigeons in age 1.5 - 4 years originated from holding on west of Slovakia, which for three months completed eighteen races at a distance of 120-1150 km. Clinical examination of health status was performed according to Scullion et Scullion.13 Faecal samples, swabs of the cloaca and oropharynx were collected from all 80 pigeons at the beginning of race season (may). On the end of race season (july) were taken samples of faeces and swabs from 69 pigeons because 11 pieces were lost during the races.
Diagnostic and laboratory analysis
Diagnostic of ornithosis complex was performed according to Rupipper8 and Smith14 based on the evaluation of clinical signs such as: rhinitis, conjunctivitis, eye wiping, nasal discharge, feather loss around the eye, epiphora, sinusitis, coughing, sneezing, fluffing and poor race performance. The floatation technique was used for detecting coccidiosis and endoparasitosis from faecal samples according to Dranzoa et al.,15 and Stenzel et al.,16 The coccidial oocyst count interpretation is shown in Table 1.
Oocysts per gram of faeces |
Interpretation |
Less than 3,000 |
Normal |
3,000 - 20,000 |
Moderate infection - performance will be affected |
20,000 - 50,000 |
Severe infection - birds may show clinical disease |
More than 50,000 |
Very severe infection |
Table 1 The coccidial oocyst count interpretation from faecal samples
Microscopic determination of swabs from the oropharynx and crop to demonstrate the presence of trichomonads was performed according to Rupipper et al. [9] The presence of ectoparasitosis caused by Columbicola columbae, Campanulotes compar, Hohorstiella lata and Menacanthus stramineus on the skin, flight and tail feathers was performed according to Smith14 and Hooimeijer17 (Figure 3). Each sample was cultivated on conventional nonselective media (blood agar) and selective media Endo agar, Staphylococcal medium N°110, Baird-Parker agar, Edwards Medium, Mac Conkey Agar (Oxoid, (OXOID Ltd., Basingstoke, Hants, UK) according to our previous studies Zigo et al.,4,18 and enterococci were identifield according to the criteria of Facklam and Collins.19 For the detection of lactobacilli were swabs of the cloaca and oropharynx put into the acidified MRS broth kept in an ice box and transported to the laboratory. The sample tubes were incubated at 37°C for 24 h. Each sample was inoculated into MRS medium (BTL, Poland) supplemented with 0.05% (w/v) Cysteine hydrochloride (Sigma-Aldrich, Poland) (MRS-cys). The plates were incubated at 37 °C for 48 h in 5% CO2. Typical colonies grown on MRS medium and only catalase-negative Gram-positive rods were considered as presumtively belonging to the genus Lactobacillus. Utilization of carbohydrates by lactic acid bacteria was assayed using API kit (API 50 CHL, bioMėrieux, France), and the results were analyzed using the APILAB Plus software (Ver. 3.3.3; bioMėrieux, France) (Figure 3).
Statistical analysis
Statistical analysis was performed using software Chi quadrate test for comparison of the most common diseases of pigeons and isolated strains from swabs of the cloaca and oropharynx before and after race season. Differences were considered as significant at the level of 0.05 or less.
There are many factors that influence the severity and scope of disease in racing pigeons. Healthy, well maintained birds will suffer lower morbidity and mortality immune compromised birds. Some factors that increase the pathogenicity of opportunistic diseases include: malnutrition, overpopulation (birds lower on the pecking order are more susceptible), suboptimal climate conditions within the loft, combining different age groups in the same loft, adding new pigeons to the flock, stressful situations within the flock, and racing birds that are not in proper condition. Diseases and vectors such as salmonellosis, trichomoniasis, heximitasis, mites, lice and endoparasites, can be immunosuppressive and increase morbidity and mortality of contagious diseases. According to Balicka et Pilarczyk10 and Shinde et al.,20 the most common diseases pigeons include coccidiosis, trichomoniasis and respiratory infections, which is also confirmed in our study. After race season were increased (P≤0.05) incidence of trichomoniasis, coccidiosis, ectoparasitosis and respiratory syndrome (Table 2).
Diseases |
Before race |
After race |
||
n |
% |
n |
% |
|
Coccidiosis |
9 |
11.3a |
36 |
52.2b |
Trichomoniasis |
6 |
7.5a |
22 |
31.9b |
Respiratory syn3 |
0 |
0a |
5 |
7.2b |
Ectoparasitosis1 |
0 |
0a |
4 |
5.8b |
Endoparasitosis2 |
0 |
0 |
2 |
2.9 |
Table 2 The most common diseases of pigeons in the monitored flock during race season
Scullion21 focused on clinical manifestations of various pigeon diseases in his work. He describes that the incidence of coccidia (Eimeria spp.) Confirmed in faecal samples does not always have a clinical course, rather the subclinical course observed in these individuals. On the contrary, the clinical manifestations of trichomoniasis increase with increasing occurrence of Trichomonas spp. isolated from oropharynx swabs. Except for trichomoniasis and coccidiosis was respiratory syndrome very common diseases in monitored flock. Respiratory syndrome is the major cause of poor performance and pigeon loss during the race season. Young birds under stress are most at risk of contracting respiratory diseases, although healthy old birds can fall ill when exposed to respiratory diseases in the race basket. Race birds with respiratory infection can be difficult to detect and yet, like a human athlete with flu, cannot compete. Clinical respiratory infection in pigeons is the end result of the interplay of a number of factors but, in particular, the type of infective organism and the vulnerability of the birds to infection are important. The classic clinical symptoms of respiratory infections include mucous in the throat, open beak and heavy breathing, rasping or gurgling while breathing, watery discharge from eyes, sometimes associated with swelling in the eye area.7
Other symptoms include discharge from the nasal area and occasionally air sac swelling or crop swelling as torn air sacs trap air under the skin. As is usually the case with pigeons, other diseases can quickly manifest themselves when birds are in distress, so other symptoms can occur, such as loose, greenish droppings and loss of weight.5,7,8 A common problems during the transport and feeding of pigeon are mixed endoparasitosis infections.4 Mixed infections with intestinal nematodes and coccidia were found in 42% of domestic pigeons and in 14.3% of the wild. As can be seen from the research, parasitic infection was greater in domestic pigeons than in the wild.7 In our study before race season we recorded low level of coccidiosis without endoparasitosis and ectoparasitosis. After the race season were increased (P≤0.05) incidence of coccidiosis and endoparasitosis. In Istanbul (Turkey), in feral pigeons nesting in famous mosques, mixed infections of coccidia and nematodes were detected: Capillaria obsignata – 19.3% and Ascaridia columbae – 14.6%. Moreover, mixed infections were described in other regions of Turkey: E. labbeana and E. columbarum were found in wild pigeons, which were infected in 15.1%.11 The incidence of diseases in pigeons during the racing season may affect the presence of bacterial microflora. Microbial flora of the pigeon gastrointestinal tract is characterized by occurrence of enterococci and E. coli. Escherichia coli is usually commensal, but can also act as an opportune pathogen. Several factors are needed for E. coli to cause disease in pigeons, such as stress or adenoviralor herpesviral infection.22,23
In our study the enterococcal and streptococcal intestinal flora of pigeons was dominated from cloaca and oropharynx swabs The predominat gram-positive bacterial strains of both collections from swabs of cloaca are Ent. faecalis, Ent. columbae, Ent. faecium, Ent. gallinarum, and Str. faecalis (Graph 1). In an increased number (P<0.05) were isolated CNS (S. epidermidis, S. schleiferi, S. sciuri), CPS (S. aureus, S. gallinarum, S. intermedius), Str. faecalis, E. coli, and Ent. columbae after race season from cloaca and oropharynx swabs (Graph 1&2).
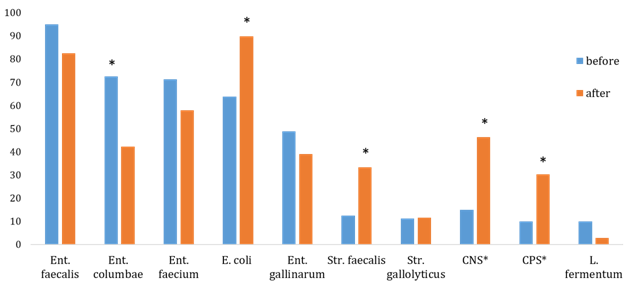
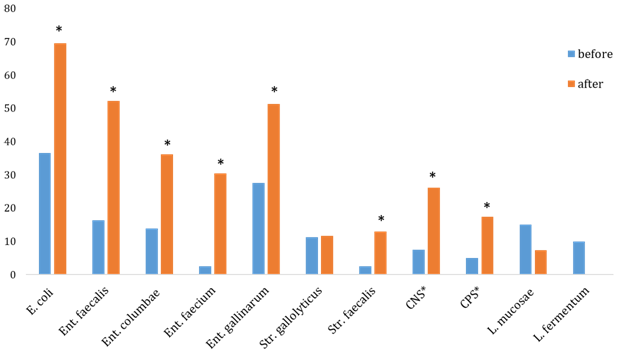
Bergmann24 describes the problem of many breeders, which is the green faeces appearing in pigeons on arrival from races. These individuals have a green faeces despite excellent performance. Soon, their overall health deteriorates rapidly, vomiting occurs, and absorption of nutrients in the intestine is impaired. The author describes the high load of pigeons during the race due to low ambient temperatures. When performing cloacal swab it was found there was a presence of E. coli and Enterococcus spp. The bacteria Str. faecalis, Str. gallolyticus with CNS and CPS are present in the intestines of normal pigeons but can also act as an opportune pathogens for immuno depression as a result a number of stress conditions, which leads to substantial exhaustion of birds and increases their susceptibility to various diseases.4,18
Serious symptoms can arise when the bacteria travels through the intestine-blood barrier. Typical is the sudden death of a pigeon which seemed completely healthy a few hours previously. This has to do with the fact that the bacteria, once in the blood, can very quickly cause serious inflammation in organs and muscles. By experimental infection of healthy pigeons whereby the Str. gallolyticus was injected directly into the blood, all the pigeons died within a few hours of being injected. Antimicrobial treatment after being infected couldn’t prevent the swift death either.7
The changes of bacteria microflora and most common diseases affecting flight speed and duration racing pigeons are related to management. Lack of population control, inadequate training, improper nutrition, and marginal preventive medicine strategies remain the common denominators in poorly managed pigeon aviaries.
Many factors will affect the performance of racing pigeons. Results are showing that also despite increased percentage of cultivation opportune pathogens and increased prevalence of coccidiosis, trichomoniasis and respiratory syndrome, the pigeons are able to provide flight performance and cope with changes in their organism also during this difficult race period. Maintaining sound husbandry practices and preventive medicine principles is vital to flock health. Many of these diseases can be prevented with proper management. In those flocks that require veterinary assistance, intervention can provide a rapid diagnosis, effective treatment, and enhanced performance.
None.
Author declares that there is no conflict of interest.

©2019 Zigo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.