eISSN: 2373-6372


Case Report Volume 5 Issue 8
1General Surgery Department, Portugal
2University of Porto Medical School, Portugal
3Instituto de Patologia e Imunologia Molecular da Universidade do Porto (IPATIMUP), Portugal
Correspondence: J Costa Maia, Sao Joao Medical Center, General Surgery Department, Portugal
Received: August 29, 2016 | Published: December 30, 2016
Citation: Morais M, Almeida MD, Eloy C, Melo RB, Graça L, et al. (2016) A Case of Mistaken Identity… Gastroenterol Hepatol Open Access 5(8): 00177. DOI: 10.15406/ghoa.2016.05.00177
Paragangliomas are rare tumors of the autonomic nervous system, which may origin from virtually any part of the body containing embryonic neural crest tissue.
A 60year-old old female, with a history of resistant hypertension and constitutional symptoms, was hospitalized for acute renal failure. In the investigation, a CT scan revealed a 63x54mm hepatic nodule in the caudate lobe. Intraoperatively, the tumor was closely attached to segment 1, but not depending directly on the hepatic parenchyma or any other adjacent structure, and it was resected. Histology reported a paraganglioma. Postoperative period was uneventful.
A potentially functional PG was mistaken for an incidentaloma, due to its location, interrelated illnesses and unspecific symptoms. PG may mimic primary liver tumors and therefore should be a differential diagnosis for tumors in this location.
Paragangliomas (PG) are rare tumors of the autonomic nervous system. Their origin takes part in the neural crest cells, which produce neuropeptides and catecholamines.1 In 10-50% of cases, they are hereditary, beginning at younger ages. In 80-90% of abdominal PG and 5-10% of head and neck PG, catecholamine production can cause arterial hypertension, headache, palpitations, anxiety and weight loss.1,2 The diagnosis is usually made by conventional imaging (CT, MRI, scintigraphy). A biopsy cannot distinguish benign from malignant tumors (<10%), which can be diagnosed in the presence of distant metastasis.3 On the other hand, biopsy of these lesions might be dangerous, with the possibility of causing a life threatening event.4 The most sensitive and specific pre-operatory diagnostic test is the determination of plasma and urine metanephrines.5 Histological examination showing polygonal cells with finely granular eosinophilic cytoplasm, oval nuclei and positivity for neuroendocrine markers such as cromogranine A, neuron-specific enolase, synaptophysin and insulin-like growth factor II are specific for paragangliomas and are not present in hepatocellular tumors.6 The treatment for non-metastatic disease involves surgical resection.5 It is also recommended that these patients must be tested for succinate dehydrogenase mutations.
In the non-cirrhotic liver, the most common benign diseases are hemangioma, focal nodular hyperplasia (FNH), nodular regenerative hyperplasia and hepatocellular adenoma. The most frequent malignancies are secondary tumors, cholangiocarcinoma and fibrolamellar carcinoma.7
Elevated αFP diagnoses hepatocellular carcinoma. Ultrassonography or CT identify cysts, metastases and hemangioma. MRI is helpful in the diagnosis of focal fatty liver, FNH, hepatocellular adenoma and hemangioma. When the diagnosis is unclear, fine needle aspiration biopsy or follow-up imaging is considered.
A 60year-old female with a history of Diabetes mellitus type 2, under treatment with sitagliptin and metformin and severe arterial hypertension treated with 4drugs (nebivolol, lecarnidipine, olmesartan and hydrochlorothiazide), was admitted to the Internal Medicine Department due to gastroenteritis and dehydration-associated acute renal failure (ARF). She reported weight loss (more than 15%), anorexia, asthenia, polydipsia, polyuria and frequent episodes of muscle cramps, with 1year of evolution. She presented no risk factors for chronic liver disease. She was the mother of 4, one of them with Cushing's syndrome. An abdominal ultrasound and angio-CT was performed for the investigation of renal failure. These exams revealed a non-cirrhotic liver, with a heterogeneous hepatic nodule of 63x54mm in the caudate lobe. This nodule had a heterogeneous contrast enhancement and included some areas of low density and enhancement (suggesting necrosis) (Figure 1). Liver function tests and tumor markers (CEA, CA19.9 and αFP) were assessed and unremarkable. Abnormal liver function could be present in the context of cirrhosis or obstructive jaundice and a raise in tumor markers could be interpreted in the context of primary or secondary neoplastic disease of the liver. Upper and lower GI endoscopies were performed to exclude digestive primaries, which showed no endoscopic lesions. Surgical resection was proposed due to location-related difficulties in obtaining a pre-operative biopsy.
Intraoperatively, after complete mobilization of the left hepatic lobe, the tumor was found closely attached to hepatic segment 1. It did not depend directly on the hepatic parenchyma or any other adjacent structure, such as the inferior vena cava, adrenal gland or kidney. The tumor was resected with no intraoperative incidents.
The nodular lesion of 7x5x5,5cm and 74g exhibited a thin capsule and smooth surface. Histology revealed a neoplasm with an expansive growth pattern, constituted by very large epitheliod cells. Some of those cells were multinucleated, with granular and amphophilic cytoplasm, and were constituted by pleomorphic nuclei with macronucleoli. Some areas of peripheral necrosis were found. The mitotic index was very low. Adrenal parenchyma was not observed (Figure 2). Immunohistochemical study revealed cromoganine expression in the absence of expression of AE1.AE3, which was consistent with the diagnosis of paraganglioma.
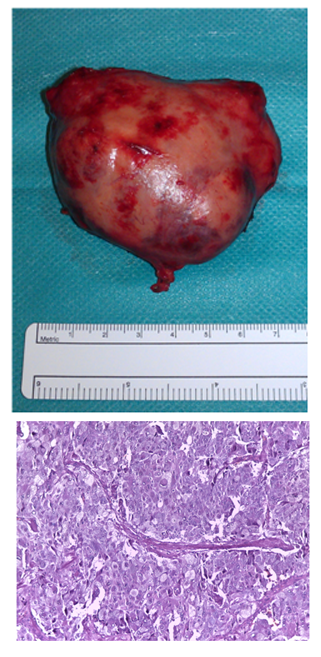
Figure 2 Macroscopic aspect (A) and histological features (HeE, 200x) (B), consistent with the diagnosis of paraganglioma.
The postoperative period was uneventful and the patient was discharged on day 5, with only one antihypertensive drug (lisinopril 20mg/day). Two months later, a cervico-thoraco-abdominal-pelvic CT confirmed the absence of any suspicious cervical nodes of PG, adrenal glands without significant changes and no retroperitoneal, mesenteric, pelvic or groin lymphadenopathies. Scintigraphy also showed no evidence of any lesion bearing α-adrenergic receptors. In addition, catecholamines in urine (vanillylmandelic acid, norepinephrine, epinephrine, dopamine, normetanephrine and metanephrine) were normal.
PG are more often located in the sympathetic chains from the neck to the pelvis. However, unusual locations, such as the gallbladder, bile ducts and bladder have been described.8-10 and in some reports, they may also mimic primary liver tumors.11,12 Tumors located in the liver are extremely rare and only few cases are described.11,13‒17 This is the first case found in literature of a PG mimicking a primary liver tumor of segment 1.
Autopsy studies have shown that, during life, 9.9% of people may develop a hepato-adrenal fusion, resulting from loss of fibrous tissue,18 which could explain the emergence of these lesions. They may be associated with the presence of ectopic cromaffinic tissue in the liver.16 This location may also be responsible, according to some authors, for the absence of symptoms. Since the venous drainage of the tumor to the portal system causes catecholamines to be metabolized by hepatocytes, their levels might not be elevated in the systemic circulation.12
In this case, the location, associated illnesses and unspecific symptoms have rendered the preoperative diagnosis of PG more difficult. Moreover, PG and hepatocellular carcinoma may have similar imaging features.4 In this case, a primary tumor outside the liver was first excluded. Then, due to the large size and location of the lesion in segment 1, which could pose difficulties for biopsy, surgical resection was proposed. This PG was an incidentaloma, which is the form of presentation in 25% of the cases.19 However, after a second-look on this case, the lesion was most probably an overlooked functional PG, due to its presentation with resistant hypertension, constitutional symptoms and also the higher prevalence of functional PG in the abdomen.
None.
Authors declare that there are no conflicts of interest.
None.

©2016 Morais, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.