eISSN: 2379-6367


Research Article Volume 7 Issue 4
1Life Sciences Department, College of Science and Technology, Al-Quds University, Palestine
2Faculty of Pharmacy, Al-Quds University, Palestine
3Department of Earth and Environmental Sciences, College of Science and Technology, Al-Quds University, Palestine
4IFBV-BELHERB, Luxembourg
Correspondence: Saleh Abu-Lafi, Faculty of Pharmacy, Al-Quds University, Abu-Dies, Palestine, Tel 972-2-2799360
Received: July 23, 2019 | Published: August 13, 2019
Citation: Akkawi M, Abu-Lafi S, Abu-Remeleh Q, et al. Phytochemical screening of Pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm Pharmacol Int J. 2019;7(4):193-200. DOI: 10.15406/ppij.2019.07.00251
The effect of wild Pomegranate (Punica granatum) juice, peels, leaves and membranes water infusion extracts on β-hematin inhibition was investigated in-vitro using semi-quantitative method. Reversed phase HPLC coupled with photodiode array detector and mass spectrometer (RP-LC-PDA-MS) was used to screen the major phenolic phytochemical(s) that may play a key role in the mechanism of β-hematin inhibition. Eighteen compounds were detected of which six are present in major quantity namely corilagin, brevifolin carboxylic acid, galloyl-HHDP-hexoside, granatin B, ellagic acid and eschweilenol C. The leaves, peels and membranes extracts have shown significant antimalarial potential when compared to chloroquine positive control. However, plain pomegranate juice which does not further enriched was inactive.
Keywords: pomegranate antimalarial, β-hematin, HPLC-PDA-MS, chloroquine, hemozoin
Malaria is a parasitic life-threatening disease that has been causing the death of millions around the globe. The latest World Malaria Report of 2018 stated that in 2017, an estimated 219 million cases of malaria occurred worldwide.1 Moreover, Plasmodium falciparum is the most prevalent malaria parasite mainly in Africa, accounting for 99.7% of estimated malaria cases. Plasmodium vivax however is the predominant parasite in the Americas, representing 74.1% of malaria cases.1 Nowadays, the most widely used synthetic antimalarial drugs such as chloroquine, amodiaquine, artemisinin derivatives and sulfadoxine/pyrimethamine (SP), have dramatically lost their efficacy because some strains of P. falciparum have demonstrated high level of resistant.2 Therefore, this has drawn more attention to the urgent need for the development of new novel drugs to treat malaria. During the past ten years, an extensive in-vitro β-hematin inhibition screening on edible food, wild natural plant extracts and/or pure isolates have been engaged in our laboratory.3‒11 The mechanism that links antimalarial effects with the ability to inhibit β-hematin formation has been thoroughly discussed.3‒11 Preparative chromatography and liquid chromatography coupled to mass spectrometer (LC-MS) were used to isolate and identify active phytochemicals present in these foods and/or natural herbs. Although encouraging results were achieved, the ability of the above-mentioned tests to predict activity against in-vivo human malaria has not yet been established.
The initial in-vitro accumulated results demonstrate that the active phytochemical(s) in edible food or natural herb may play an efficient role in improving the health of malaria infected patient in the future. Pomegranate, Punica granatum (Punicaceae) for example is a very rich source of a broad array of phytochemicals that belong to different chemical classes such as phenolic acids, flavonoids, and tannins, etc.12 Besides sharing many of the same compounds within the different parts of pomegranate plant such as juice, peel, membranes and leaves, many other diverse phytochemicals are found. Juice and extracts from different parts of pomegranate fruit have been shown to exhibit different therapeutic activities including for inflammation, cardiovascular disorders, cancer, diabetes, male infertility, Alzheimer, etc.12 The antioxidant, anticancer, anti-inflammatory, antidiabetic and antimicrobial properties of pomegranate were extensively reviewed recently.13
The main phytochemicals present in pomegranate have already been separated and identified. In the literature, there are several papers that report about pomegranate extracts constituents using liquid chromatography coupled to mass spectrometry particularly using HPLC-PDA-ESi-MS methodology.14‒17 The main aim of this work is first to separate and identify the main components in pomegranate extracts using LC-PDA-MS. Then to measure the effect of the water extracts derived specifically from juice, peel, membranes and leaves on β-hematin inhibition using the semi-quantitative methods developed by Deharo et al.18
Plant collection
Fresh pomegranate fruits were collected from Bani-Naim village in the Hebron city, southern part of Palestine. External peels and membranes were air dried in the shade for 25 days. Leaves were dried in the laboratory at 37oC for a week. A sample of fresh juice was prepared through crushing the fruit manually.
Materials
HPLC grade solvents of acetonitrile (ACN), dimethyl sulfoxide (DMSO), chloroquine diphosphate salt, hemin chloride were all purchased from Sigma-Aldrich. Highly purified water was prepared using a Millipore Milli-Q plus water purification system.
Extraction of the secondary metabolites of pomegranate
Dried samples of the peel and membrane were cut into small pieces and homogenized in an electric mixer until a smooth texture was obtained. Leaves sample was ground into powder. Fresh juice sample was filtered by (MN 615.Ø110) filter paper. Two grams of these samples "except the fresh juice" were added separately; to 150 ml of distilled water at 90- 95ο C, left for about 15 minutes at room temperature. The extract was then filtered using (MN 615.Ø110) filter paper. Then, it was lyophilized using Labconco freeze drier (USA), until constant weight was achieved. The final dried extract was stored in an amber bottle and kept in the fridge.
In-vitro semi-quantitative method
The developed method of Deharo et al was adopted in this work.17 The solutions were added to the plate in the following order. First a mixture containing 50μL of 0.5mg/mL hemin chloride freshly dissolved in dimethyl sulfoxide (DMSO), second a 100μL of 0.5M sodium acetate buffer (pH 4.4), third 50μL of potential anti-malarial drug solution or solvent, was incubated in a non-sterile well flat bottom plate at 37ºC for 18-24 h. It is important that the solutions be added to the plate in this order. The plate was then centrifuged for 10 minutes at 4000 rpm. The supernatant was removed and the pH of reaction was measured. The final pH of the mixture was between (5.0-5.2). The wells were washed with 200μL DMSO per well to remove unreacted free hemin chloride. The plate was centrifuged again followed by discharging the supernatant. The β-hematin remaining was then dissolved in 200μL of 0.1M NaOH to form an alkaline hematin that can be measured spectrophotometrically at 405nm using ELISA reader. Ultra-pure water was used as negative control while chloroquine was used as the positive control and the tested extracts were dissolved in ultra-pure water.
Instrumentation
The analytical HPLC is Waters Alliance (e2695 separations module), equipped with 2998 Photo Diode Array (PDA). Data acquisition and control were carried out using Empower 3 chromatography data software (Waters, Germany). In parallel, the analyses were performed on a Waters Acquity UPLC H-Class system (Waters, Milford, MA, USA) equipped with a binary solvent manager, sampler manager, column manager, Acquity QDa detector and PDA connected to Waters Empower 3 data station. An Acquity UPLC BEH C18 column (50mm x 2.1mm I.D., 1.7μm) equipped with an Acquity BEH C18 1.7μm guard column (Vanguard 2.1 x 5mm) was also from Waters, Milford, MA, USA).
Chromatographic conditions
The HPLC analytical experiments of the crude water infused extracts were run on ODS column of Waters (XBridge, 4.6 ID x 150 mm, 5 μm) connected to guard column of Xbridge ODS, 20 mm x 4.6mm ID, 5μm. The mobile phase is a mixture of 0.5% acetic acid solution (A) and acetonitrile (B) run in a linear gradient mode. A 100% (A) was descended to 70% (A) in 40 minutes then to 40% (A) in 20 minutes and then to 10% (A) in 2 minutes and stayed for 6 minutes and back to the initial conditions in 2 minutes. The HPLC system was equilibrated for 5 minutes with the initial acidic water mobile phase (100 % A) before injecting next sample. All the samples were filtered with a 0.45mm PTFE filter. The PDA wavelengths range was from 210-500nm. The flow rate was 1ml/min. Injection volume was 20ml and the column temperature was set at 25◦C. Samples were filtered through 0.45mm micro porous disposable filter.
The LC-MS column and sample temperature were maintained at 30˚C and 20˚C respectively. The mobile phase consisted of 0.1 % formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a constant flow rate of 0.4mL/min. The linear gradient starting conditions was 90% A, increased to 60% B over 12 minutes. Then it increased to 100% B in one minute and stayed there for two minutes. Prior any injection the mobile phase was equilibrated for 4 minutes. The injection volume was 1μL. The detection range of the PDA was from 210-500nm. The Acquity QDa ionization modes were in the negative and positive ESi, mass range was between 100-1000 Da.
Chromatographic profiles of pomegranate extracts
The method of choice for extracting phytochemicals from food or natural herbs in our laboratory is infusion.3‒11 Since different preparations may exert different actions and safety profiles, we opt to use it the most popular and simple method. Infusion crude water extracts of pomegranate leaves, juice, peels and membranes were freshly prepared and directly analyzed using HPLC-PDA and the UV absorption was high at 350nm (Figure 1). The majority of the eluted peaks were possessing absorbing maxima in the range between 219-224, 242-275 and 328-377nm.
Although each extract has its unique HPLC chromatographic profile, however, the four extracts share many similar phytochemicals at different proportions (Figure 1). To explore the identity of the separated peaks, RP-LC-PDA-ESi-MS mode was used in this study. The previously published LC-MS structural identifications based on high resolution mass spectroscopy were beneficial to us to tentatively identify the main compounds separated in the four pomegranate extracts.14‒17 Figure 2, for example, shows the LC-PDA-MS chromatogram at 260 nm of pomegranate water leaves extract. Table 1 shows the deprotonated [M-H]- derived pseudo negative ions from pomegranate leaves that we were able to tentatively identify based on previous published papers (Table 1).
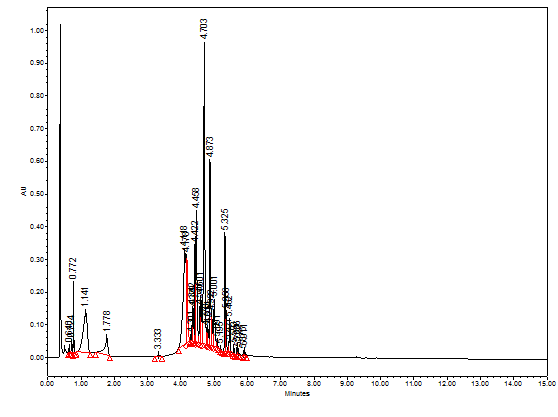
Figure 2 LC-PDA-MS chromatogram at 260 nm of pomegranate water leaves extract. The overlaid UV-Vis spectra of the peaks are shown in the figure.
|
# |
RT (minutes) |
[M-1]- Da |
Tentative name |
|
1 |
0.51 |
191 |
Quinic acid |
|
2 |
0.642 |
331 |
Galloyl-hexoside |
|
3 |
0.722 |
169 |
Gallic acid |
|
4 |
0.779 |
343 |
3,4,3'-Tri-O-methylellagic acid |
|
5 |
1.141 |
331 |
Galloyl-hexoside |
|
6 |
1.778 |
432 |
unknown |
|
7 |
4.170 |
634 |
Corilagin |
|
8 |
4.118 |
291 |
Brevifolin carboxylic acid |
|
9 |
4.3 |
506 |
unknown |
|
10 |
4.422 |
633 |
Galloyl-HHDP-hexoside |
|
11 |
4.458 |
633 |
Galloyl-HHDP-hexoside |
|
12 |
4.703 |
951 |
Granatin B |
|
13 |
4.873 |
301 |
Ellagic acid |
|
14 |
5.150 |
610 |
Cyanidin-3,5-diglucoside |
|
15 |
5.325 |
447 |
Eschweilenol C |
|
16 |
5.462 |
447 |
Eschweilenol C |
|
17 |
5.593 |
523 |
Secoisolariciresinol hexoside |
|
18 |
5.688 |
417 |
Kaempferol 3-O-b-D-Xyloside |
Table 1 Phytochemicals from water extract of pomegranate leaves using LC-ESi-MS in the negative mode
There are hundreds of phytochemicals belonging to different families reported in pomegranate.14‒17 However, using the infusion extraction method, only eighteen compounds were observed of which six are major. Figure 3 depicts the chemical structure of some of the compounds present in pomegranate leaves. Figure 4 shows selected MS spectra of phenolics in the negative ESi mode (Table 1). Gallic and ellagic acid are among the compounds separated in pomegranate extract (Table1). Researchers have previously mentioned the antimalarial activity of gallic acid and some of alkyl ester gallate.19 Moreover, the in-vitro antimalarial activity of ellagic acid was also reported by other researchers.20
In-vitro Semi-quantitative β-haematin inhibition method
The results of the in-vitro semi-quantitative experiments on the four infusion extracts are shown in comparisons to positive and negative controls in Figures 5-8. As can be seen, the absorption is inversely proportional to the extract efficiency, i.e., the lower the absorption, the better is the extract efficiency in prevention β-hematin formation. The fresh pomegranate juice extract was completely inactive towards β-hematin inhibition (Figure 5).
The external peels and leaves extracts were highly efficient in inhibiting β-hematin formation (Figures 6 & 7) and slightly more active in comparison to chloroquine positive control. The peel extract has better efficiency at 100% and 50% dilutions in spite of a good result at 30% concentration, but it became less efficient at 20% concentration (Figure 6). This result is expected since efficiency decreased as a result of dilution. Leaves infusion extract showed an excellent efficiency; the best activity appears even at very low dilution of 10%. The efficiency of the leaves extract is better than the peel, according to figure 6 and 7 to which the absorbance of the peel extract at 20% is 0.145, while it is 0.055 at the same dilution level.
Figure 8 shows the effect of membrane extract in β-hematin inhibtion in-vitro. The 100% and the 50% solutions gave less effect in comparison to the positive control.The mechanism of action between the pomegranate extract phytochemicals and the synthetic β-hematin which is spectroscopically identical to the native pigment is not clear yet. Nonetheless, since most of the pomegranate phytochemicals are rich with hydroxyl, carbonyl and sometimes carboxylic acid functionalities, an energetically favored complex between the phytochemical(s) with ferriheme may led to the formation of a stable complex which prevent β-hematin formation. The interaction presumably formed between the Fe (III) metal center with one of the carbonyl groups of the pomegranate phytochemicals, whereas, the side chain carboxyl group of the heme may interact with one of the many hydroxyl groups of this specific phytochemical (Figure 3). Other mechanistic studies also believed that the ability to form p-p complexes may strongly bind hematin monomers thus inhibiting their association.21
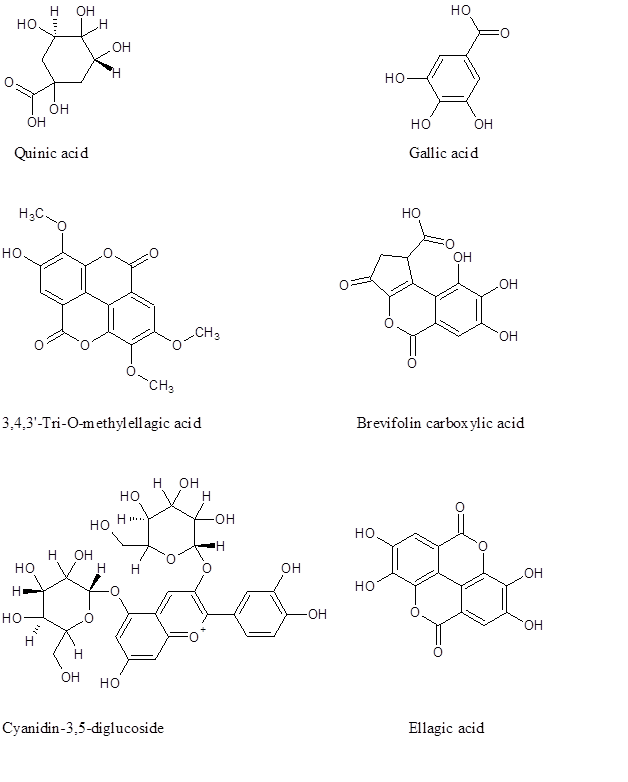

Figure 3 Chemical structure of some of the existed phytochemicals in the infused leaves of pomegranate.

Figure 4 (-)-ESi-MS spectra of some of the phenolics appeared in Table 1, A, Galloyl-hexoside; B, Ellagic acid; C, 3,4,3'-Tri-O-methylellagic acid; D, Granatin B
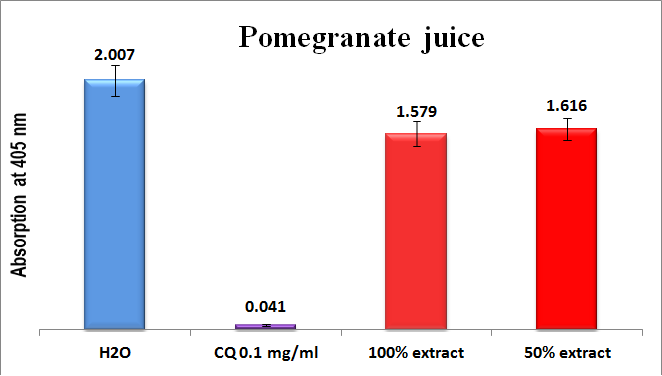
Figure 5 Column diagram representing antimalarial efficiency (absorption of dissolved β-hematin at 405nm) of pomegranate juice in comparison to negative (water) and positive chloroquine (CQ-0.1mg/ml) controls. Each result represents an average of 16 individual experiments.

Figure 6 Column diagram representing antimalarial efficiency (absorption of dissolved β-hematin at 405nm) of water extracts of pomegranate peel in comparison to negative (water) and positive chloroquine (CQ-0.1mg/ml) controls. Each result represents an average of 16 individual experiments.
Wild pomegranate infusion water extracts of peels, leaves and membranes showed encouraging in-vitro antimalarial activity compared to chloroquine positive control. Surprisingly, pomegranate plain juice extract (not the concentrated one) was inactive. Isolating pure phytochemicals from these extracts and testing them against chloroquine reference control may pave the way for new novel compounds with promising antimalarial activity. Further studies are to be conducted comprising more diverse pomegranate fruits from different locations.
None.
The authors declare that there is no conflict of interest.

©2019 Akkawi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.