eISSN: 2379-6367


Research Article Volume 6 Issue 4
1Life Sciences Department, Al-Quds University, Palestine
2Faculty of Pharmacy, Al-Quds University, Palestine
3Department of Earth and Environmental Sciences, Al-Quds University, Palestine
4Centre for Chemical and Biological Analysis, Al-Quds University, Palestine
5IFBV-BELHERB, Luxembourg
Correspondence: Saleh Abu-Lafi, Faculty of Pharmacy, Al-Quds University, Abu-Dies, Palestine , Tel 972-2-2799360
Received: June 18, 2018 | Published: July 2, 2018
Citation: Akkawi M, Abu-Lafi S, Abu-Remeleh Q, et al. HPLC separation of phenolic phytochemicals from grape peels and seeds water extracts and their invitro antimalarial activities. Pharm Pharmacol Int J. 2018;6(4):253-259. DOI: 10.15406/ppij.2018.06.00183
Water extracts of three grape types (black, shami and white) were separated using reversed phase HPLC-PDA and their in-vitro effect on β-hematin inhibition were investigated. The grape crude water extracts of peels and seeds impede the formation of β-hematin in vitro and therefore possess a significant antimalarial effect. Black grape peel extract gave superior activity as revealed by its absorption value in comparison to CQ positive control. In general, black and shami peels water extracts were slightly more active than their corresponding seeds extracts while surprisingly, the white grape peel extract was completely inactive. The same inactivity was noticed when black, shami and white grape juices as well as red and white wines were examined in-vitro. The lack in white grapes of the active phytochemicals that present at high levels in black and shami grapes explains the inactivity of the former. Several secondary plant phenolic metabolites may be responsible for the antimalarial activity and subsequently, one could infer that the antimalarial activity of water extract may be a result of the synergistic effect of its diverse phenolic phytochemicals.
Keywords: HPLC, grape, seeds, peels, antimalaria, β-hematin, chloroquine, hemozoin
According to WHO statistics, malaria is considered one of the most prevalent infectious diseases that is responsible for killing half a million humans annually. In 2016, about 216 million cases of malaria were estimated worldwide, of which 445,000 deaths were reported globally. Most malaria cases were in Africa and accounted for 91% of all malaria deaths.1 Malaria is caused by a parasite which is transmitted to humans through the bite of infected female of Anopheles mosquito followed by introduction of the parasite into the human host bloodstream. Generally, it is caused by one of four protozoa of the genus Plasmodium, namely, P. malariae, P. ovale, P. vivax and P. falciparum.2 In sub-Saharan Africa, P. falciparum is the most prevalent malaria parasite, accounting for 99% of estimated malaria cases in 2016.1
Digestion of hemoglobin by the malaria parasite leads to the liberation of large amounts of free heme known as ferriprotoporphyrin IX. Detoxification of the free heme occurs by conversion into brown pigment crystals called hemozoin. The formation of hemozoin is vital for survival of the parasite making it a major target of action for numerous antimalarial drugs. Synthetic crystalline analogue to hemozoin is called β-hematin and used in assay determination for antimalarial compound screening in-vitro.3‒8 The growing evidence of parasite resistance to frontline prescribed antimalarial agents such as chloroquine (CQ), antifolates and artemisinin has highlighted the urgent need to develop novel antimalarial drugs to treat this vicious disease. Many of the current antimalarial drugs were originally derived from natural sources; a huge chemical diversity of secondary metabolites exists in nature. The effect of pure isolated compounds, crude extracts and preparative HPLC fractions of selected natural products on β-hematin inhibition was recently reported9‒15 based on semi-quantitative assay protocol developed by Deharo et al.,16 for screening of antimalarial activities.
Naturally occurring secondary metabolites of phenolic phytochemicals from different grape types have gained more attention due to their health-promoting and antioxidant potentials.17 Phenolic type and composition depends mainly on the grape variety used and the adopted extraction methodology. Soluble phenolic compounds are usually extracted by using binary ethanol-water solvent mixture (80% ethanol-20% water). Important phenolic compounds in grape include simple aromatic rings with low molecular weight to complex high molecular weight tannins. Important monomeric phenolic compounds in grapes are hydroxybenzoic acids (e.g., gallic acid, vanillic acid, syringic acid, etc), hydroxycinnamic acids (p-coumaric acid, caffeic acid, etc), Stibens (trans-resveratrol), Flavonols (kaempferol, quercetol, myricetol, etc), flavan-3-ols (catechin, epicatechin, gallocatechin, epigallocatechin) and anthocyanins (cyanidin-3-O-glucside, delphindin-3-O-glucside, paeonidin-3-O-glucside, petunidin-3-O-glucside, malvidin-3-O- glucoside).17 Proanthocyanidins are class of complex polyphenols (dimers or oligomers of catechin and epicatechin and their gallic acid esters) that are present in black and red grapes and called tannins.17 Grapes are also rich in anthocyanins which give the black, red and purple grapes their distinctive dark color but are not found in white grapes. Anthocyanins accumulate mainly in the peels whereas procyanidins are located in the seeds.18 This work aims to analyze the phenolic phytochemicals present in different local Palestinian grape types (black, shami and white) extracts of seeds, peels and in juices. Screening of their antimalarial efficacy was achieved by using a semi-quantitative assay in vitro.
The solvent dimethyl sulfoxide (DMSO, 99.5% purity), chloroquine diphosphate salt, sodium acetate (99% purity) and hemin chloride were procured from Sigma-Aldrich. Glacial acetic acid was purchased from Fluka. Ethanol (EtOH) solvents were purchased from Merck (Germany). Three types of Palestinian grapes, namely, shami (red colored), black and white colored grapes were bought from a local market in Jerusalem. Samples were air-dried in the shade for about a week, grinded and kept in the refrigerator until use.
Preparation of plant extract
Infusions were obtained by soaking 2-grams of the grape in 150 ml of distilled hot water at 90°C for 20 minutes, then left to cool down at room temperature, then filtered using MN 615 Ø110mm filter paper. Dried, concentrated extracts from the infusions were obtained by evaporation of the water at 60-70°C under reduced pressure using a rotary evaporator (IKA WEREKRV06-ML), followed by freeze-drying (Labconco freeze drier) until a constant weight was achieved. The final dried extract was stored in opaque bottles and kept in desiccators until analysis by HPLC.
In-vitro semi-quantitative assay for screening of antimalarial activity
The protocol adopted in the current study was the one developed by Deharo et al.,16 In brief, a freshly prepared mixture containing 50μl of 0.5mg/ml haemin chloride (in DMSO), 100μl of 0.5M sodium acetate buffer at pH 4.4 and 50μl of the tested anti-malarial drug solution or control, was incubated in a normal non-sterile 96-well flat bottom plate at 37ºC for 18-24hours. It is imperative that the solutions be added to the plate in this order. The plate was then centrifuged for 10 minutes at 4000rpm. The supernatant was removed and the pH of reaction was measured. The final pH of the mixture should be between 5.0-5.2. The wells were washed with 200μl DMSO per well to remove free haemin chloride. The plate was centrifuged again, discharging the supernatant afterwards. The β-haematin remaining was then dissolved in 200μl of 0.1M NaOH to form ferricprotoporphyrin IX that could be measured spectrophotometrically. The absorbance was then determined at 405nm using an ELISA reader (Stat Fax-2100). Milli-Q ultrapure water was used as negative control, whereas chloroquine dissolved in ultrapure water was used as positive control.
Chromatographic conditions
Grape water extracts (5mg/ml each) was run on reversed phase ODS column of Waters (XBridge, 4.6IDx150mm, 5μm) with a guard column of XBridge ODS (20mmx4.6mm ID, 5μm). The mobile phase consisted of a linear gradient mixture of a solvent A (0.5% acetic acid solution) and solvent B (acetonitrile) with a flow rate of 1mL per min. The gradient was started with 100% of solvent A and adjusted for 70% of solvent A and 30% of solvent B in 40minutes; 40% of solvent A and 60% of solvent B in 20minutes; 10% of solvent A and 90% of solvent B in 2minutes and remained there for 6minutes and then back to the initial conditions in 2minutes. The HPLC system was equilibrated for 5minutes with the initial acidic water mobile phase (100% A) before injecting the next sample. All the samples were filtered with a 0.45μm PTFE filter. The PDA wavelengths range was from 210-500nm. Injection volume was 20μl and the column temperature was at room temperature.
Results of the in-vitro semi-quantitative experiments made on different grape types are depicted in Figures 1-7. The antimalarial efficiency as reflected by the absorption of the dissolved β-haematin at 405nm of water extracts of black, shami and white grapes peels and seeds as well as red and white wine in comparison to negative (water) and positive control (CQ-0.1mg/ml) is depicted in Figure 1. The absorption is inversely proportional to extract efficiency and to hemozoin content, i.e., the lower the absorption, the more efficient the extract and the stronger the inhibitory effect towards hemozoin formation. Although the exact mechanism of action of the grapes as antimalarial extracts is not recognized exactly at this stage, it is evident however, that some of the extracts were strongly inhibited the formation of β-hematin. As in Figure 1, out of eleven different water extracts, only five showed encouraging results that are comparable or better than CQ positive control. Black grape peel extract gave superior activity as revealed by its absorption value (0.104) in comparison to (0.107) value of the CQ positive control (Figure 1). In general, black and shami peels water extracts were slightly more active than their corresponding seeds extracts while surprisingly, the white grape peel extract was completely inactive. The same observation was noticed when black, shami and white grape juices as well as red and white wines were examined in-vitro.
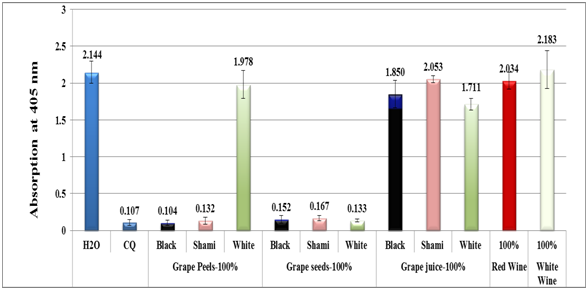
Figure 1 Column diagram representing antimalarial efficiency (absorption of dissolved β-hematin at 405nm) of water extracts of black, shami and white grapes peels, seeds, juices as well as red and white wine in comparison to negative (water) and positive (CQ-0.1mg/ml) controls. Each result represents an average of 16 individual experiments.
Assuming the activity of grape extracts such as black peel act via similar mechanism to that of CQ positive control, i.e., through the formation of a complex between certain phenolic compound(s) of grape water extract and ferriheme, the complex generated prevent the formation of β-hematin. The lack in white grapes of the same active phytochemicals present at high levels in black and shami grapes may explain the inactivity of white grapes. Black and red grapes are rich in flavan-3-ols, flavonols, stilbene, anthocyanins and tannins. Subsequently, one could infer that the antimalarial activity of grape water extract may be as a result of the synergistic effect of its diverse rich phenolic phytochemicals.
Figure 2 shows the column diagram of the antimalarial activity of black peel water extract at different dilutions using distilled water which leads to different concentrations of the crude black peel extract in comparison to negative and positive control (CQ-0.1mg/ml). The antimalarial activity was maximum at 100% concentration while it kept decreasing with dilution. At 5% the activity it was two folds less than at 100% level.
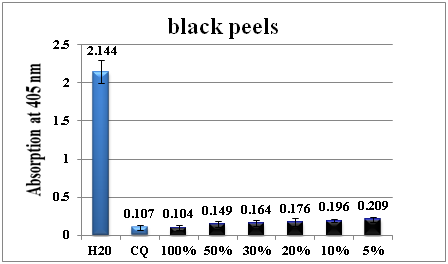
Figure 2 Column diagram representing the antimalarial efficiency of black peel water extract compared with the negative (water) and positive control (CQ-chloroquine 0.1mg/ml), showing the absorption values of dissolved β-haematin (alkaline haematin) at 405nm. Each result represents the average of 16 individual experiments.
Figures 3‒7 show column diagram representing the antimalarial efficiency of different grape water extract dilutions leading to different concentrations compared with the negative (water) and positive control (CQ-0.1mg/ml), showing the absorption values of dissolved β-haematin (alkaline haematin) at 405nm. Each result represents the average of 16 individual experiments.
In Figure 3, the activity of the shami grape peel water extract at 100% concentration showed its maximum (absorption of 0.132), and it remained almost in insignificant decline up to 20%. Afterwards, at 10%, the antimalarial activity dropped 5 folds and subsequently sharply decreased at the 5% concentration level. The white grape peels water extract were inactive at all concentrations used (Figure 4).

Figure 3 Column diagram representing the antimalarial efficiency of shami (red) peel water extract compared with the negative (water) and positive control (CQ-chloroquine 0.1mg/ml), showing the absorption values of dissolved β-haematin (alkaline haematin) at 405nm. Each result represents the average of 16 individual experiments.
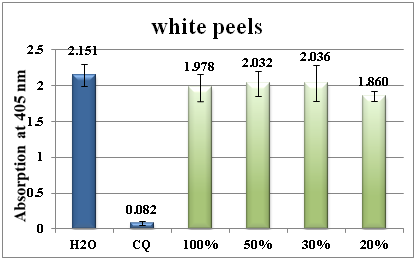
Figure 4 Column diagram representing the antimalarial efficiency of white peel water extract compared with the negative (water) and positive control (CQ-chloroquine 0.1mg/ml), showing the absorption values of dissolved β-haematin (alkaline haematin) at 405nm. Each result represents the average of 16 individual experiments
The antimalarial activity of the black seeds extract remains active even at 5% concentration (Figure 5). Similar observations were made for shami and white seeds extract (Figure 6) (Figure 7).
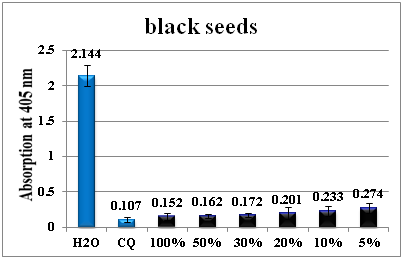
Figure 5 Column diagram representing the antimalarial efficiency of black seeds` water extract compared with the negative (water) and positive control (CQ-chloroquine 0.1mg/ml), showing the absorption values of dissolved β-haematin (alkaline haematin) at 405nm. Each result represents the average of 16 individual experiments.
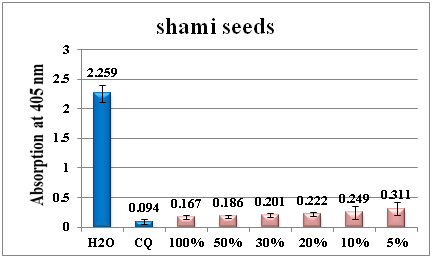
Figure 6 Column diagram representing the antimalarial efficiency of red shami seeds water extract compared with the negative (water) and positive control (CQ-chloroquine 0.1mg/ml), showing the absorption values of dissolved β-haematin (alkaline haematin) at 405nm. Each result represents the average of 16 individual experiments.
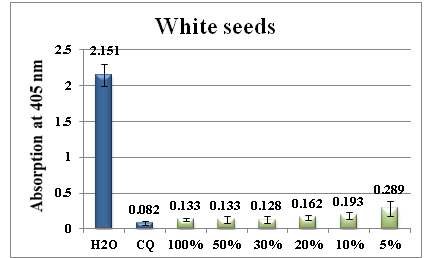
Figure 7 Column diagram representing the antimalarial efficiency of white seeds water extract compared with the negative (water) and positive control (CQ-chloroquine 0.1mg/ml), showing the absorption values of dissolved β-haematin (alkaline haematin) at 405nm. Each result represents the average of 16 individual experiments.
Figure 8 shows the chromatogram of the crude water soluble extract of the antimalarial active black grape peels at 350nm. The 350nm wavelength was selected because the main peaks eluted at 9.1, 21.7, 24.0, 24.6, 27.4, 29.4 and 66minutes exhibited a maximum absorption near to this wavelength between the range of 312-356.9nm. As shown in Figure 8, about 5 major peaks and some other minor compounds showed the dominance of phenolic phytochemicals as revealed by their UV-Vis spectra. The LC-MS and UV-Vis maximum absorption of grapes phytochemicals are well documented in the literature.19‒21 As seen in Figure 8, the peak eluted at 9.1minute showed lmax at 325.8nm, a typical absorption of caffeic acid which belongs to hydroxycinnamic acid.19‒21 Moreover, the peak at 21.23minutes exhibited a lmax at 350.9nm which is most probably a quercetin-3-O-rhamnoside flavonoid.20 The two close by eluted compounds at 24.0 and 24.6 minutes possess the very same spectra and indicating to their structurally related nature. They have lmax at 353.3nm most probably signifying to the presence of quercetin-3-O-glucoside (isoquercetin) and quercetin-3-O-glucuronide (miquelianin) flavonoids.19‒21 The last lipophilic eluted peak at 66minutes has a lmax at 356.8nm which may due to syringetin 3-(6-acetyl) glucoside. 19‒21
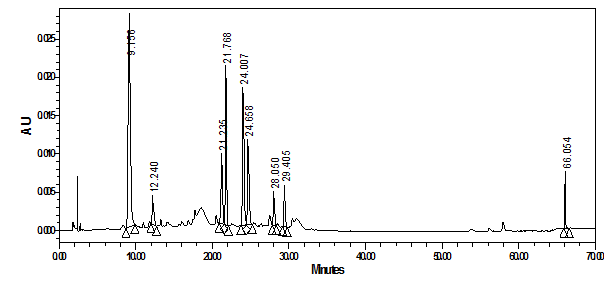
Figure 8 HPLC-PDA chromatogram of crude water extract of black grape peels at 350nm and their overlaid UV-Vis spectra.
At the same monitoring wavelength of 350nm, Figure 9 shows the chromatogram of the crude water soluble extract of shami grape peels. Using the same concentration of grape shami extract, the main peaks gave less response as a result of their lower concentration in the extract. While the peak at 21.7minutes (quercetin-3-O-rhamnoside) was disappeared, another polar peak at 2.78minute emerged with a lmax of 325.8nm indicative most probably to astilbin flavanonol.20
Figure 10 shows the chromatogram of the crude water soluble extract of white grape peels. As expected, the peaks responses were much lower in comparison to Figure 8 and Figure 9. New peak eluted at 11.14 minutes (lmax of 311.4nm) assigned tentatively as p-hydroxybenzoic acid, the phenolic derivative of benzoic acid.19,20 In general, it should be emphasized that relatively similar secondary metabolite profiles were detected in the different grape peel extracts but at different concentrations (Figures 8‒10).
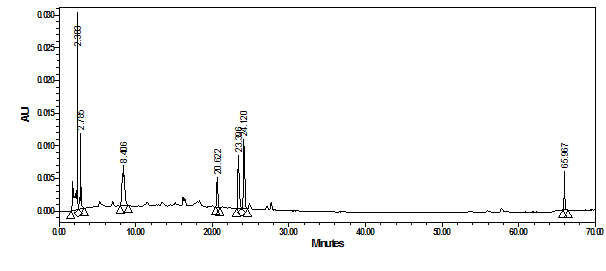
Figure 9 HPLC-PDA chromatogram of crude water extract of shami grape peels at 350nm and their overlaid UV-Vis spectra.
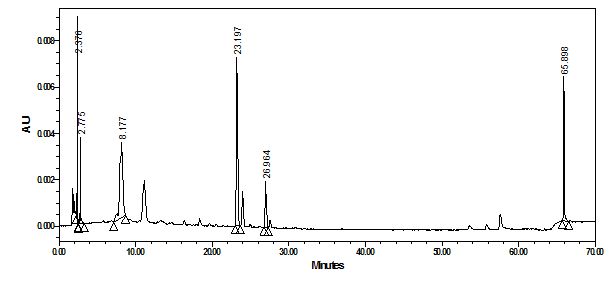
Figure 10 HPLC-PDA chromatogram of crude water extract of white grape peels at 350nm and their overlaid UV-Vis spectra.
As to the seeds extracts of the same 3 grape types, suitable monitoring wavelength was chosen at 275nm since no absorption was seen above 280nm. Figure 11 showed that the major eluted peaks of shami grape seeds were not as those seen in shami grape peels and their retention times and UV-Vis spectra were different.
Figure 12 & Figure 13 represent the peak profile of black grape and white grape seeds at 275nm respectively. The peaks at 11.8, 13.29, 15.5, 17.1, 18.6, 19.5, 23.3 and 64.3minutes showed lmax between 276.9-279.3nm. Comparing acquired wavelengths to literature indicates the absence of gallic acid since the maximum absorption of gallic acid is about 272nm.19‒21 Moreover, 279nm absorption points to epicatechin or catechin compound, while 276 and 277nm absorptions indicate to epi-gallocatechin-epi-catechin and epi-catechin-3-O-gallate respectively.19,20
The complexity of the rich phenolic metabolites of grape make it hard to conclude which compound(s) is responsible for the antimalarial activity and therefore, the antimalarial activity of grape water extract may be due to synergistic effect.
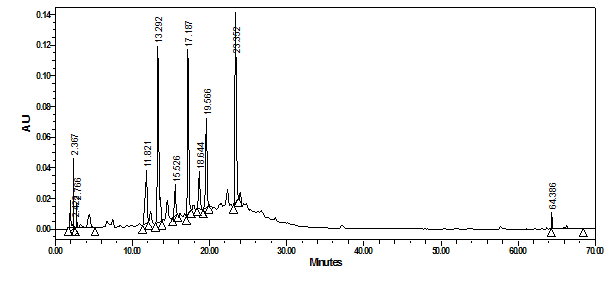
Figure 11 HPLC-PDA chromatograms of crude water extract of shami grape seeds at 275nm and their overlaid UV-Vis spectra.
The results showed that water extracts of Palestinian grapes seeds and peels have strong antimalarial activityinvitro. Several phenolic secondary metabolites were found in grapes seeds, peels at different concentrations using by HPLC-PDA. The grape fruit plant deserves more attention by subjecting it to more fractionation, isolation and identification using LCMSMS methodologies and testing these fractions or pure compounds for antimalarial efficiency in vitro.
None.
The author declares that there is no conflict of interest.

©2018 Akkawi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.