eISSN: 2379-6367


Research Article Volume 7 Issue 5
Biochemistry Department, School of Pharmacy, Istanbul Medipol University, Turkey
Correspondence: Ozan Emre Eyupoglu, Biochemistry Department, School of Pharmacy, Istanbul Medipol University, Kavacık South Campus, Goztepe Dist. Ataturk Str. No.40, 34810, Beykoz-Istanbul/Turkey, Tel +90538 588 20 70
Received: September 11, 2019 | Published: October 8, 2019
Citation: Eyupoglu OE. Measurements and monitorings dependent color intensity of drugs and food supplements interactions with textile dyes between TLC plates by using rope printing technique. Pharm Pharmacol Int J. 2019;7(5):238-243. DOI: 10.15406/ppij.2019.07.00257
Pharmacovigilance between drug and food supplements is common in the world and it is important to take precautions before clinical cases. In this study, interactions between food supplements containing herbal food mixes, sweeteners, vitamin blends and commonly used painkillers, antibiotics and anticoagulants, were revealed with helping of macrame ropes, smartphone, TLC plates and textile dyes and color analysis method supported image j program. The sensitivity of the developed method was high (LOD: 0.7ppm, LOQ: 2ppm). Color analysis results can provide a color risk scale that can be combined with online drug interaction softwares such as Micromedex.
Keywords: drug-food interaction, textile dyes, rope printing, color intensity analysis, TLC plates
The discussions of pharmacokinetic and pharmacodynamic interactions between drugs and medicinal plants has been put forward, especially with medicinal herbs that can interact with antidiabetic drugs. In addition to their treatment, many diabetic patients are known to use herbal medicines that have both antidiabetic effects and potential benefits.1 Approximately 72.8% of people with diabetes use herbal medicine and dietary supplements.2 Interactions between herbal mixtures and medicines may increase the effectiveness of antidiabetic agents. For example, antidiabetic drugs have been shown to increase blood glucose-lowering effects with agrimony.3 Many anti-diabetic drugs are substrates of the CYP450 enzyme system, and many medicinal plants may also affect this system. For example, ginkgo inhibits CYP3A4, CYP2C9 and CYP2C19, while St John's wort inhibits CYP2C and CYP3A.4 Some of the commonly used antidiabetic drugs include pancreatic beta-cell receptors, α-glucosidase inhibitors, e.g., acarbose, peroxisome proliferator activated receptor activators, e.g., thiazolidindiones.5 Many of these plants which include bio-active molecules such as peptides, alkaloids, lipids, terpenoids, amines, sulphur compounds, coumarins, steroids, flavonoids, and inorganic ions, have been used in traditional medicine as antidiabetics.6 As the use of herbal medicines increases, short-term or long-term toxicity due to side effects, overdose, hypersensitivity can be detected by pharmacodynamics or pharmacovigilance.7 In 2010, as a good example practice, the Upsala monitoring center created a database of 4 million reports on about approximately 21000 herbal products from 100 countries in the World.7 In the US, herbal products are classified as botanicals or dietary supplements, not medicines. In Europe, the definition of a herbal product as a food or medicine may have a significant effect on pharmacovigilance, as there is no legal requirement for food supplements.7 Classifications of adverse reactions of herbal medicines in orthodox medicine were formed as Type A (acute); dose-dependent, Type B (specific); not dose-dependent, Type C (chronic): cumulative effect, Type D (onset); genotoxic, carcinogenic.8
Adverse effects reports were generally about unaware of the using of herbal and nutritional products by physician or patient.9 There have been an extensive list of herbal remedies used in hospital environments in Thailand.10 Consumers and industries can report adverse events related to the use of dietary supplements via the electronic forms MedWatch 3500, 3500A and 3500B, an adapted safety reporting portal by FDA.11 For example, it has been possible that mixtures of pharmaceuticals containing aristolochic acid from Chinese and Western pharmaceutical components may cause kidney damage, and mixtures of drugs containing tripterygium glycosides may cause reproductive system disorders and mixtures of Polygonum multiflorum from the buckwheat family may cause liver damage.12 Unexpected adverse effects from digitalis, garlic, senna extract, mustard oil, menthol, aloe, and turmeric plant mixtures have been reported.13 With comparative pharmacoepidemiology, prospective, meta-analysis, retrospective studies, case control and cohort studies, the safety of herbal medicines has been investigated by testing the signals detected from the self-reporting program systems.14 The scope of drug-herbal food studies should be extended to include drug use review, photochemical analysis, prescription sequence symmetric analysis, quality monitoring, quantification of components and non-clinical safety studies.15 Organic anion carriers containing more than 10 transmembrane transport proteins have great potential in many dietary supplements-drugs, plant-endogenous compounds, drug-drugs, plant-drug interactions. Organic anion carriers play an important role in the excretion and detoxification of many water-soluble drugs, compounds or additives (eg: nonsteroidal anti-inflammatory drugs, neurotransmitter metabolites, mycotoxins, phenolic acids and flavonoids) from the kidneys and liver, respectively.16 Both in vitro and in vivo studies have shown that some flavonoids may have the ability to regulate organic anion transporter (OAT) activity (eg, Apigenin inhibits OAT1 activity while catechin inhibits OAT4). Flavonoids present in most plants may cause plant-drug interactions in combination with antiviral and antibiotics.17 The natural sweetener stevioside and its aglycone metabolite steviol reduce the renal clearance of some anionic drugs as they show a high affinity for both OAT1 and OAT3.18
The use of multiple drugs and dietary supplements is very common, especially in AIDS and cancer patients.19,20 A mixture of dextromethorphan, midazolam (oral and intravenous administration), tolbutamide and caffeine were administered to healthy people for evaluation of intestinal and hepatic activities by using a cocktail approach and a greater effect was observed in the small intestine than the liver.21 Consumption of large amounts of grapefruit juice, apple juice or orange juice reduces plasma levels of fexofenadine (antihistamine), an organic anion carrier substrate in the intestine, by inhibition according to in vitro evaluations.22
The British Drug Safety Committee reported five cases of warfarin anticoagulation interacting with cranberry juice.23 Red yeast rice, obtained by fermenting Monascus purpureus yeast on rice, and was developed as a dietary supplement to lower blood lipids, could increase the dosage of verapamil.24 Pharmacists and health professionals should educate patients appropriately to minimize possible adverse juice-drug interactions.25
Standardised questionnaires were used to determine risky uses of herbal medicines and dietary supplements in cancer treatment.26 More than half of the reported 108 herb supplement-drug interaction cases were sourced of green tea (13.9%), garlic (14.8%), ginger (3.7%), mistletoe (9.3%), Chinese herbs (8.3%). The most commonly reported drugs that interact with herbal supplements were cyclophosphamide, irinotecan, warfarin, nonsteroidal antiinflammatory drugs, paclitaxel and vinorelbine. For example, garlic, combined with aspirin and omeprazole, increased the risk of gastrointestinal bleeding. The interactions between green tea supplements and irinotecan, warfarin or cyclophosphamide were also possible.27 Catechin and caffeine compounds in green tea inhibited the release of arachidonic acid from platelets and inhibited thromboxane and clot formation. Dietary supplements such as Marshmallow, Barley, Iceland Moss, Rice Bran, Coffee Charcoal, Quince could reduce the absorption of warfarin.28 Mania induction was observed in depressive patients who mixed panax ginseng and antidepressants, while Chinese herbal product, xaio chai hu tang (sho-saiko-to), caused a decrease in blood concentrations together with prednisolone. Gummy soluble fibers could reduce the absorption of drugs.29 In a cell culture experiment, Caco-2 cell monolayers containing hepatocytes were used to investigate the in-vitro effects of various components (curcumin, hesperetin, quercetin, naringenin, and piperine) in irinotecan transport and the study was followed by in vivo.30 Potential interaction of warfarin with vitamin E, coenzyme Q, ginkgo, ginseng, papaya, garlic, Devil’s clam, Danshen, Dong quai, fish oils increased international normalized ratio, while potential interaction of warfarin with St. John’s wort and green tea decreased international normalized ratio.31
Oral anticoagulants, containing Factor Xa inhibitors (rivaroxaban, apixaban and edoxaban), whose safety profile is superior to vitamin K antagonists, which are more interacted with herbal supplements or foods, have begun to replace warfarin.32 Some of the anti-retroviral agents used for the treatment of HIV (eg; ritonavir, nevirapine, saquinavir, efavirez) inhibit CYP3A4 enzymes and interact with warfarin, is a drug with narrow therapeutic index, to diminish its effectiveness.33 Royal jelly which is a thick, milky white food produced by bees by worker bees (Apis mellifera L.) contraindicates against acute asthma exacerbation, eczema, shortness of breath, hemorrhagic colitis, runny nose, conjunctivitis, and atopy, facial edema, can cause a possible bleeding risk by interacting with warfarin.34 Calcium-supplemented foods increase antibiotic resistance and create therapeutic failure. Malnutrition may also affect the absorption of drugs. The absorption of oral drugs by the intestine is usually in competition with nutrients. Therefore, for example, diuretics can cause side effects on urinary potassium loss as well as impaired cardiac function.35 Alcohol intake causes increased renal excretion of folate and poor absorption of niacin, vitamin C, thiamine, vitamin A and vitamin B6.36
Foods such as carbonated soft drinks, tea, chocolate, and coffee containing methylxantane may interact with medications and create deep and undesirable effects on the young central nervous system.37 The interaction between an anti-depressant and vitamin B complex intake may have a potential effect on the central nervous system.38 Curcumin increases the glutathione S-transferase activity while valerian reduces uridine diphosphoglucoronosyl transferase activity. Due to these enzymatic effects, drug plasma levels are adversely affected.39 Medications used in the last 2 weeks of life for 166 adult patients under palliative care were screened for potential interactions by using the ‘Stockley’s Drug Interactions On-line 10th Edition’ software with clinical pharmacists. Decreasing body mass and decreasing renal and hepatic clearance for these patients may help to tolerate side effects of drugs.40 Interactions between herbal supplements and analgesic, anti-cancer drugs related to palliative care have been documented. Detected supplement-drug interactions that increase the risk of potential bleeding included cod liver oil and diclofenac, garlic and ibuprofen, cranberry juice and warfarin.41 Drug interaction software such as online Lexi-Interact and Micromedex are widely used by prescribers to identify clinically meaningful drug interactions because interactions between several drugs can be controlled at the same time.42 Despite the hepatoprotective effects of nutraceuticals such as chlorogenic acid, syringic acid, eriodictyol, caffeic acid, ferulic acid, naringenin, resveratrol, quercetin, rosmarinic acid, it has been reported that they can adversely affect the efficacy and toxicity of analgesics and antipyretic drugs, containing acetaminophen.43 Since it is important to develop different techniques to detect all these interactions before they become clinical cases, the work on this paper can be valuable. In spite of the fact that thin-layer chromatography (TLC) plates have been useful for quantitative analysis of pharmaceutical products and routine qualitative analysis of plant extracts,44 the use of TLC plates in combination with the developed rope printing technique to investigate drug-herbal food supplement interactions in vitro is not available in the literature. For this purpose, in this work, the ropes dipped in different drug-food supplement liquid mixtures were dyed with textile dyes and then printed between TLC plates and the power of interaction (synergist and antagonist side effects) was rated by measuring the color intensity of these prints with helping of Image J, a color analysis program (Figure 1).
All textile dye reagents were solved with ultrapure water produced by using Milli-Q® IQ 7003/7005 Ultrapure Lab Water System (Merck). Dylon trademark Green-Fabric Dye and Tulip Red Fabric Dye were purchased from Migros shopping market, Turkey and prepared by adding to 150 mL mixtures of hot ultrapure water and table salt, separately for rope dyeing. Green Chain vegetable powder (135 g, 30 sashe), contains 46 kinds of herb mixes such as Ginkgo biloba, green tea, garlic extracts and more, was obtained from The LifeCo Company, Turkey. A solution of 4.5 g of a powder mixture was prepared by stirring with IsoLab magnetic mixturer in 150 mL of warm water. Orzax Ocean Multi Vitamin Mineral Fish Oil Syrup (food supplement flavored with honey and orange aroma, 150 mL), Supradyn All Day (30 Tablet) vitamin and mineral supplement, Aspirin® (Acetylsalicylic acid) 100 mg, (20 Tablet), AKSEF 500 mg (cefuroxime axetil) (10 tablet) and Coumadin (Warfarin Sodium) 10 mg, (28 tablet) drugs were purchased from pharmacy, Turkey for used as interaction samples. A solution of one tablet from each drugs in 150 mL of warm water was prepared separately. TLC Silica gel 60 F254 Aluminium sheets (20 x 20 cm) were purchased from OrLab® laboratory market in order to create the printing surface. YarnArt 50 g beige cotton macrame rope (5 mm thick and single auger) was purchased from haberdashery, Turkey to be used in dyeing and printing. With an imaging system consisting of an Apple iPhone 8 64GB smartphone webcam and a multivariate image analysis program (Image J), print images between TLC plates reflecting the intensity of interaction after dyeing with textile dyes of ropes dipped in different drug-food supplement mixtures were depicted by using as inputs of partial least squares with helping plot chart of color values for red (R), green (G) and blue (B) parts of the images. Red and green color rope print lines were produced between TLC plates and Images of rope print lines were photographed with helping a smartphone. The zone area lines of images were imported in software to compute the color pixel densities proportional to the severity of drug-food supplement interactions (Figure 1).
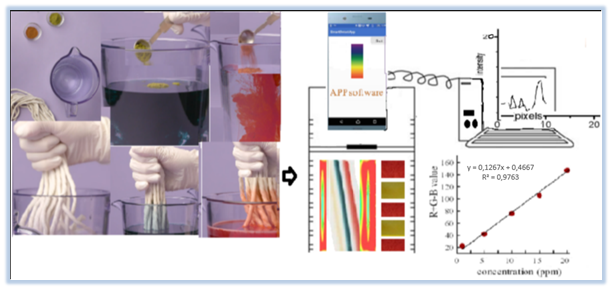
Figure 1 Color-analysis flow diagram of drug-food supplement interaction after rope staining and TLC printing.
In addition, function of MATLAB with chemometric methods was used in combination of matrix values with number of pixels. While pure black or white or unused blue color areas were specified with triple matrix code ‘’[R=G=B=0]’’, the others were specified with binary matrix code ‘’[R=G=1]’’. For the image color quality, % average relative prediction error was determined based on the distance between the TLC plate and the mobile phone webcam. If this error affects red and green color depth, it is indicated by -1 matrix code.45 With good precision and accuracy, high sensitivity, statistical validation parameters of this improved method which is one similar of computational quantification tests using image,46 were measured and regression curve data of this improved method were performed by using Microsoft Excel (Microsoft Office Corporation, 2010, Redmond, Washington) and SPSS Version 21.0 software program. All statistical analysis were reported significantly (p< 0.05) with standard deviation. The limit of detection (LOD) and limit of quantification (LOQ) were determined by the average of standard deviation of the calibration curves by created via Zoner Photo Studio 17 program in terms of 3 times and 10 times of rate of ‘slope / standard deviation’ obtained from the calibration graphs of color line areas of all drug-food supplement interactions respectively in which the smallest pixel color depth parameters (with CIELAB (L*, a*, b*, h°, K / S) color measurement method47) of rope printing images on TLC plates painted with textile dyes taken with the help of smartphone. Colour intensity values may be transformed into a pseudo absorbance value and then plotted against concentration to obtain linearized plots.48
Detection of colorless molecules based on reversible and rapid color change is an example of molecular recognition as it is based on strong molecular structures and shapes. Cetirizine, one of the most commonly antihistamine drugs, used in the treatment of allergy, competes with phenolphthalein, a color indicator to form an inclusion complex with β-cyclodextrin.49
Visible absorption spectra of coal tar dye spots in commercial cosmetics were measured with a reversed phase TLC plate by using a scanning densitometer.50 The color density analysis of a yellowish brown complex formed by passing arsine gas through a filter paper pre-soaked in mercury bromide solution was found by scanning the image and calculating with the help of software.51 In a study, photo color analysis measurements under light source of wool fabrics dyed with pomegranate peel, madder and alkanet root were shown with angle and tint color parameters (CIELAB (L*: Light-dark difference, a*: Red-green difference, b*: Yellow-blue difference, h°: Light angle) and ratio of absorption and scattering coefficients (K/S).52 In an other study, for detecting the SO2 concentrations of twenty-five practical food samples, practical assay platform including paper chip combined color analysis application installed on a smartphone was developed.53 Subtle color and shape changes caused by sulfur dioxide in the air in moss leaves were detected by a simple webcam and imaging processing algorithm to observe the risks of chemical exposure through the plant.54 To quickly detect formaldehyde in food samples, a simple paper-based analytical device (PAD) was developed based on the reaction of formaldehyde with excess sulphide to form sodium hydroxide and its acid-base titration with phenolphthalein color indicator and via using of a digital camera.55
The λmax values of microgram amounts of dyes (Victoria Blue R, methylene blue and fluorescent) were measured on TLC plates by using new sample application discs and TLC scanner.56 In this study, Calibration graphs of the color intensities of the TLC line fields in the captured images were created (Figure 1). Detection limits (LOD: 0.7 ppm, LOQ: 2 ppm) were respectively determined as 3 times and 10 times of the average ratios (slope/standard deviation) obtained from the calibration graphs with triplicate smartphone applications. Linear range was found between 0.5 ppm-3 ppm (Table 1). For intra-day and inter-day conditions, the differences in color intensity measurements between the wet or dried dyed images and standard errors due to close and distant filming to images of TLC plates were indicated with a matrix value of -1 in the triple color matrix. All results were statistically significant (p< 0.05). Green and red color densities were expressed as +1 matrix value in linear rope print images on TLC plates (Table 1). Before identical macrame ropes were dipped into the 0.01 sashe green chain vegetable powder aqueous solution and into half tablet Aspirin® (Acetylsalicylic acid) aqueous solution separately, it was dipped into red and green fabric dye solutions and pressed between TLC plates. After identical macrame ropes were dipped into the 0.01 sashe green chain vegetable powder aqueous solution and into half tablet Aspirin® (Acetylsalicylic acid) aqueous solution separately and dried for 30 seconds separately, dyeing and pressing processes were repeated between TLC plates.
|
Sample Interactions |
Matrix color codes |
CIELAB Pixel color depth |
|||||||
|
(x1) green |
(x2) red |
(x3) blue |
L* |
a* |
b* |
h° |
K/S |
Percentage error |
|
|
Green chain powder+Aspirin |
0 |
1 |
0 |
61.27±0.02 |
3.27±0.03 |
30.42±0.04 |
85.33±0.01 |
11.71±0.03 |
0.17% |
|
Green chain powder+Aksef |
0 |
1 |
0 |
53.42±0.02 |
4.35±0.03 |
32.45±0.04 |
83.42±0.01 |
10.93±0.03 |
0.30% |
|
Green chain powder+warfarin |
1 |
0 |
0 |
55.24±0.02 |
5.16±0.03 |
34.85±0.04 |
78.65±0.01 |
9.72±0.03 |
0.50% |
|
Supradyn all day+Aspirin |
1 |
-1 |
0 |
65.19±0.02 |
2.33±0.03 |
36.48±0.04 |
93.75±0.01 |
8.75±0.03 |
0.75% |
|
Supradyn all day+Aksef |
-1 |
1 |
0 |
50.42±0.02 |
6.42±0.03 |
33.41±0.04 |
90.45±0.01 |
12.33±0.03 |
0.85% |
|
Supradyn all day+warfarin |
1 |
-1 |
0 |
45.18±0.02 |
3.18±0.03 |
29.87±0.04 |
86.40±0.01 |
7.89±0.03 |
1.00% |
|
Orzax Ocean Multi Vitamin Mineral Fish Oil Syrup+Aspirin |
1 |
1 |
0 |
47.25±0.02 |
6.85±0.03 |
31.33±0.04 |
77.33±0.01 |
6.55±0.03 |
0.25% |
|
Orzax Ocean Multi Vitamin Mineral Fish Oil Syrup+Aksef |
1 |
1 |
-1 |
49.83±0.02 |
2.79±0.03 |
28.46±0.04 |
79.45±0.01 |
15.78±0.03 |
1.20% |
|
Orzax Ocean Multi Vitamin Mineral Fish Oil Syrup+warfarin |
1 |
-1 |
-1 |
51.12±0.02 |
4.65±0.03 |
27.12±0.04 |
91.15±0.01 |
17.21±0.03 |
1.50% |
Table 1 Matrix and color depth parameters of drug-food interactions
L*, Light-dark difference; a*, Red-green difference; b*, Yellow-blue difference; h°, Light angle; (K/S), ratio of absorption and scattering coefficients. Values are means of triplicate determinations (p<0.05)
Rope printing images of TLC plates were photographed by smart phone with three repetitions. Before and after the drug-food supplement interactions, the differences in color intensities of linear prints of rope printing increased depending on the forces of the interactions with color tone clarity from Image J programme (Figure 1). In contrast, red and green fabric dye solutions interacting with identical macrame ropes dipped into 0.1 tablet AKSEF (cefuroxime axetil) and 5 tablet Coumadin (warfarin sodium) aqueous solutions, separately, decreased in color intensities of linear prints of rope printing. This manner was understood from the increase in the L* value from the pixel color depth parameters because sulfo groups in fabric dyes may interact with hydroxyl groups in warfarin and amino groups in cefuroxime axetil more than carbonyl groups in acetylsalicylic acid (Table 1). All concentrations were adjusted to a single dose (0.0003 g/mL) for easy comparison of staining color intensities with each other. While 0.1 tablet Supradyn All Day vitamin and mineral supplement aqueous solution caused the discoloration of the rope suppression of the dyes between TLC plates by interacting with Aspirin® and Coumadin drugs, it caused darkening of color with aqueous solution of 0.1 tablet AKSEF antibiotic drug. Color lightening showed negative interaction and color darkening showed positive interaction (Table 1). Orzax Ocean multi vitamin mineral fish oil syrup was negatively interacting with all medicines (Aspirin®, AKSEF, and Coumadin) because the free carboxyl group of the arachidonic acid derivative in fish oil can interact with drugs. Color scale bandages with green and red fabric dyes were applied as indicators of drug-food supplement interactions. In the all printing process between TLC plates with dyed rope, measuring of color intensity as a blind trial against water was removed from all actual color interaction trials.
In Turkey, in vitro qualitative and quantitative measurements of interactions of intensely sold commercial dietary supplements and commonly used painkillers, antibiotics, each other based on the color determination were demonstrated in this paper for the first time and in this study, proven results with color analysis can be associated with commonly used online drug interaction softwares such as Lexi-interact and Micromedex for creating risk color scales inserted drug package. This in-vitro study, which is an easy and fast applicable emergency triage without clinical experience about drug-food supplement interactions also supports in-vivo studies.
This article does not contain any studies with human participants or animals performed by the author.
I would like to thank my family for their moral support. I did not receive any financial funding or material supports interested with the article.
The author stated that there is no conflict of interest relevant to this study.

©2019 Eyupoglu. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.