eISSN: 2379-6367


Research Article Volume 11 Issue 2
1Clinical Pharmacokinetics Center, University of São Paulo, Brazil
2Plastic Surgery and Burns Division, Department of Surgery of Medical School, University of São Paulo, Brazil
Correspondence: Silvia R C J Santos, Clinical Pharmacokinetics Center, University of São Paulo, São Paulo/SP, Brazil, Tel 55 11 95357- 8930
Received: May 20, 2023 | Published: May 31, 2023
Citation: Santos SRCJ, de Camargo TV, Messiano CG, et al. Combined vancomycin-meropenem therapy in pediatric major burns undergoing therapy of septic shock guided by cultures and pharmacokinetic-pharmacodynamics approach based on serum levels to combat bacterial resistance. Pharm Pharmacol Int J. 2023;11(2):62-68. DOI: 10.15406/ppij.2023.11.00403
Subject-background: Meropenem and Vancomycin are largely prescribed to septic patients with infections caused by Gram-positive and Gram-negative nosocomial pathogens during the systemic inflammatory response syndrome. Pharmacokinetic changes reported previously in burns can impact the desired outcome. In addition, drug serum monitoring is recommended to critically ill pediatric patients with infections undergoing therapy of septic shock. Then, dose adjustment at the earlier period of septic shock must be done especially for vancomycin by applying pharmacokinetic-pharmacodynamics (PK/PD) tools based in serum levels in pediatric major burn patients. Subject of study was to investigate effectiveness of a combined therapy of vancomycin with meropenem in pediatric major burns with renal function preserved or augmented by vasopressors requirements, done in a real time guided by PK/PD approach based on serum levels and cultures of isolates.
Methods: Patients receiving vancomycin were investigated after the empiric daily dose recommended and after dose adjustment requirements. Therapy started with 40-60 mg/kg daily, one hour pump infusion, and if required dose was increased to attain vancomycin recommended target: area under the curve/minimum inhibitory concentration (AUCss0-24/MIC) > 400. Blood was sampling at the 3rd and 5th hr. of the infusion started for serum measurements done by liquid chromatography. In addition, pediatric burns receiving also meropenem were investigated after the recommended dose (40mg/kg q8h) administered by 3 hrs.-extended infusion to attain the target 100%f∆T>MIC; minimum inhibitory concentration. Blood was sampling at the 3rd and 7th hr. of the infusion started for serum levels done by liquid chromatography. One compartment open model was chosen to investigate pharmacokinetics of antimicrobials. Drug effectiveness was evaluated by PK/PD approach and the microbiology of isolates by cultures. Clinical outcome was based on antimicrobial coverage for clinical and microbiological cure guided by cultures.
Results: Septic major burn patients 42 (22M/20F) with preserved or augmented renal function were distributed by age: 2-7 yrs. (Group 1, n=11), 8-13 yrs. (Group 2, n=11), 14-18 yrs. (Group 3, n=20). Target was attained by vancomycin up to MIC 1mg/L against Gram-positive strains only in 43% (18/42) patients receiving an empiric dose regimen (10-15mg/kg q6h, 1hr infusion, Set 1). Vancomycin individualized therapy was prescribed; then, the coverage was extended against MIC 2 mg/L strains in 35/42 patients. Vancomycin effectiveness occurred in a short period by clinical cure of all patients. In addition, after meropenem therapy (40mg/kg q8h) done by 3hrs.-infusion, significant changes between groups occurred on PK parameters impacting the coverage age dependently. Clinical cure occurred also for all patients against Gram-negative strains by eradication of Gram-negative strains up to MIC 4 mg/L in a short period.
Conclusion: PK/PD target of 100%f∆T>MIC was attained by meropenem for all patient’s despite of significant PK changes between groups registered, once the desired outcome was reached; while the target (AUCss0-24/MIC>400) recommended for vancomycin effectiveness was guided by PK/PD approach based on serum levels and cultures. Dose adjustment done in a real time permits an earlier medical intervention to reach the desired clinical outcome in a short period. Then, PK/PD approach based on antimicrobials serum monitoring is an important tool to assess drug effectiveness in septic pediatric burns, and consequently to avoid microbial resistance.
Keywords: septic shock, major pediatric burns, Vancomycin-Meropenem combined therapy, PK/PD approach based on serum levels, therapy guided by culture of isolates
AUCss0-24,area under the curve; AUCss0-24/MIC>400, area under the curve: MIC ratio higher than 400 up to 600 (Vancomycin PK/PD target); 100% f∆T>MIC, time dose interval that free drug serum levels is higher than MIC (Meropenem PK/PD target); CSLI, clinical standard laboratory institute, database USA; GSA, global sepsis alliance; ICU, intensive care unit; LAIS, Latin American Institute of Sepsis; MDR, multidrug resistance; MIC, minimum inhibitory concentration; MRP4, multidrug resistance-associated protein 4; MV, mechanical ventilation; OAT1, OAT1-organic anion transporter 1; OAT3, OAT1-organic anion transporter 3; PD, pharmacodynamics; PK, pharmacokinetics; PK/PD, pharmacokinetic-pharmacodynamics; PNM, pneumonia; PTA, probability of target attainment; SAPS3, simplified acute physiology score 3; SARS-CoV-2; severe acute respiratory syndrome coronavirus 2; SOFA, sequential organ failure assessment; SSC, surviving sepsis campaign; SIRS, systemic inflammatory response syndrome; TBSA, total burn surface area; TDM, therapeutic drug monitoring; WHO, World Health Organization
Septic shock is a preventable and potentially fatal organ dysfunction caused by a dysregulated host response to infection.1 The clinical outcome in most high-risk cases is the mortality of patients including pediatrics with nosocomial bacterial infections associated viral infections as influenza and most recently SARS-CoV-2. In view of the growing challenge to the prescription of antimicrobials for adequate treatment and effective control of bacterial infectious conditions during COVID-19 pandemic, Surviving Sepsis Campaign in the International Guidelines for Management of Sepsis and Septic Shock (2021), Global Sepsis Alliance (2020), and World Health Organization (2023) have reinforced that combating bacterial resistance, and the prevention of the development of multidrug-resistant strains (MDR) is urgent.1-5
It is well known that at the last years, it was reported a significant increase in the minimum inhibitory concentration (MIC), independently of microbial database considered. In a monitoring program of antimicrobial therapy for several infections based on serum levels of beta-lactams in patients admitted to the ICU, it was reported that almost 80% of patients did not reach the therapeutic target against susceptible strains of Gram-positive strains by vancomycin and Gram-negative bacteria by meropenem. This fact reinforces that monitoring serum levels is essential to assess changes in pharmacokinetics that impact the coverage of the prescribed antimicrobial agent, expressed through the PK/PD approach. Therefore, new therapeutic strategies have been proposed for the most prescribed beta-lactam agents related to the dose regimen and duration of infusion for meropenem.6-10
Some prospective controlled studies including therapeutic drug monitoring (TDM) were considered to compare drug efficacy based on serum levels of several antimicrobial agents usually prescribed in the therapy of septic shock. Considering that therapeutic drug serum monitoring of meropenem after 3 hrs.-extended infusion strategy that ensures an adequate serum level against intermediate susceptible strains coverage up to MIC 4 mg/L, and in combating the intermediate susceptibility strains primarily related to K. pneumoniae and P. aeruginosa in major septic burns receiving vasopressors. In addition, to allowing the evaluation of vancomycin effectiveness considering the dose regimen prescribed to septic ICU patients, drug serum levels are a laboratory strategy of great value in the individualization of therapy in a real time, guaranteeing the expected clinical outcome, and to combating the development of bacterial resistance, also reducing the duration of antimicrobial therapy, and consequently hospital costs.7 It is noteworthy that, so far, serum levels of these agents are not routinely monitored in hospitals for these critically ill patients admitted to ICUs.8,9 Clinical management for critically ill patients in intensive care has been guided by cultures through the isolation of the agent followed by determination of the susceptibility of the pathogen to the antimicrobial and supported by the serum biomarkers of Systemic Inflammatory Response Syndrome. Then, if these results are combined with PK/PD approach based on serum levels, it is possible to guide antimicrobial therapy. If serum levels of the prescribed antimicrobial agent are equal to or greater than those required to eradicate the susceptible pathogens, microbiological cure will occur, and the desired clinical outcome will be achieved by appropriately treating the septic shock and healing the patient, on the other hand, for serum levels below the recommended level, therapeutic failure will occur.10-12
Subject of study was to investigate antimicrobial effectiveness of a combined therapy of vancomycin and meropenem in pediatric major burns with renal function preserved or augmented by vasopressors requirements, done in a real time by pharmacokinetic-pharmacodynamics (PK/PD) approach based on serum levels and cultures.
Study design, patient eligibility, and the combined vancomycin meropenem therapy
The clinical protocol was a prospective, open-label study. Ethical approval register CAAE 07525118.3.0000.0068, Brazilian Platform was obtained by approval of the Ethical Committee of Hospital of Clinics, Medical School of University of Sao Paulo; no conflicts of interest to declare were obtained from all authors. The study was conducted from July 2019 to December 2021, and informed written consent was obtained from all legally designated patient representatives. Pediatric patients from the Intensive Care Burn Unit higher than 2 years up to 18 years old, presenting severe thermal injury and a sepsis diagnosis as reported Greenhalgh et al. in the “American Burn Association consensus conference to define sepsis and infection in burns” in clinical evaluation and laboratorial data were eligible for inclusion.13
On the other hand, patients with vancomycin or meropenem intolerance or renal impairment were excluded. The study was based on the recommended antimicrobial treatment to suspected or documented Gram-positive and Gram-negative nosocomial infections of hospital. Thus, vancomycin meropenem combined therapy were prescribed at dose regimen recommended for pediatric patients with renal function preserved or augmented by vasopressors required at the earlier stage of septic shock. Septic major burn pediatric patients 42 (22M/20F) with preserved or augmented renal function by vasopressors requirements at the earlier stage of septic shock, were included to investigate antimicrobial effectiveness of combined therapy guided by cultures, and PK/PD tools based on drug serum levels. Patients were allocated by age in three groups: 2-7 yrs. (Group 1, n=11), 8-13 yrs. (Group 2, n=11), 14-18 yrs. (Group 3, n=20). Initial dose regimen, body weight normalized, was given 10-15 mg/kg q6h, equivalent to 40-60 mg/kg daily or 10-15mg/kg q8h, by one hour pump infusion for vancomycin in Set 1, and dose adjustment was done in Set 2, if required to individualize therapy and to achieve clinical outcome. In addition, meropenem were administered systemically by pump 3 hrs.-infusion at the dose regimen recommended, according to the institutional protocol. Complete medical histories, physical examinations were obtained for each enrolled patient; laboratory data included strains documented in blood cultures, and susceptibility testing done to obtain the minimum inhibitory concentration for each antimicrobial agent against each pathogen isolated.
Demographic, and clinical characteristics of patients at admission, and during drug serum monitoring in the Intensive Care Burn Unit (ICBU) are shown in table 1; creatinine clearance was estimated by Schwartz’s method.14
|
Demographic, Laboratorial and Clinical data, Coverage based on serum levels, Outcome - |
Group 1 (2-7yrs n=11) |
Group 2 (8-13yrs n=11) |
Group 3 (14-18yrs n=20) |
|
Gender M/F |
6M/5F |
7M/4F |
16M/4F |
|
Age (yrs) |
3(2-5) |
11(10-12) |
16 (14-18) |
|
Body weight (kg) |
16(15-18) |
40(39-55) |
70 (55-74) |
|
Height (cm) |
101(91-109) |
128(119-156) |
165 (160-171) |
|
Body surface area (m2) |
0.68(0.61-0.91) |
1.21(1.15-1.35) |
1.70(1.54- 1.84) |
|
Body mass index (kg/m2) |
18.9(18.5-20.8) |
23.0(22.3-24.4) |
23(22-24) |
|
Admission data |
|||
|
TBSA % |
23(18-39) |
31(29-35) |
36(27-38) |
|
Inhalation injury |
10/11 |
3/11 |
13/20 |
|
Thermal burn |
10/11 |
8/11 |
16/20 |
|
Electrical trauma |
1/11 |
3/11 |
4/20 |
|
Mechanical ventilation |
10/11 |
3/11 |
18/20 |
|
Vasopressors |
10/11 |
3/11 |
15/20 |
|
Biomarkers at admission |
|||
|
C- reactive protein |
90(43-179) |
39(34-132) |
252(180-289) |
|
Leucocytes (*1000 cells/mm2) |
13,81(7.49-16-98) |
7.76(3.58-14.94) |
17.10(14.20-19.20) |
|
Biomarkers at TDM |
|||
|
C- reactive protein (mg/L) |
145(81-269) |
246(182-301) |
140(97-325) |
|
Leucocytes (*1000 cells/mm2) |
14.36(10.49-17.13) |
18.05(8.74-18.93) |
16.69(14.10-21.60) |
|
Neutrophils (*1000 cells/mm3) |
13.53(8.65-13.54) |
14.85(6.77-16.61) |
13.80(11.0-18.50) |
|
Serum creatinine (mg/dL) |
0.25(0.21-0.31) |
0.32(0.23-0.42) |
0.65(0.55-0.87) |
|
Creatinine clearance (ml/min) |
240(167-285) |
282(250-296) |
177(144-205) |
|
Vancomycin dose regimen 1hr. infusion |
|||
|
Empirical dose regimen (mg/kg q6h) |
10-15 |
10-15 |
NAP |
|
Adjusted dose regimen (mg/kg q6h) |
20-38 |
20-40 |
NAP |
|
Empirical dose regimen (mg/kg q8h) |
NAP |
NAP |
10-15 |
|
Adjusted dose regimen (mg/kg q8h) |
NAP |
NAP |
18-25 |
|
PK- parameters - Vancomycin |
|||
|
Biological half-life (hrs) a[3.7-5.3 hrs] |
2.9(2.6-3.4) |
3.0(2.6-3.3) |
3.0(2.7-3.5) |
|
Volume of distributions (L/kg) a[0.45-0.65 L/kg] |
0.49(0.40-0.60) |
0.44(0.36-0.54) |
0.55(0.45-0.71) |
|
Plasma clearance: (ml/min*kg) a[1.2-1.7 mL/min*kg] |
1.70(1.53-1.94) |
1.90(1.59-2.28) |
2.11(1.88-2.61) |
|
PK/PD approach Vancomycin 1hr. infusion |
|||
|
Coverage MIC 0.5 mg/L (empirical vs dose adjusted) |
11/11 vs 11/11 |
11/11 vs 11/11 |
20/20 vs 20/20 |
|
Coverage MIC 1.0 mg/L (empirical vs dose adjusted) |
5/11 vs 11/11 |
8/11 vs 11/11 |
20/20 vs 20/20 |
|
Coverage MIC 2.0 mg/L (empirical vs dose adjusted) |
0/11 vs 7/11 |
0/11 vs 8/11 |
6/20 vs 20/20 |
|
Meropenem dose regimen 3hrs. infusion |
|||
|
Recommended dose regimen (mg/kg q8h) |
38-40 |
35-42 |
15-22 |
|
Dose regimen (mg/kg daily) |
114-120 |
105-126 |
45-66 |
|
PK- parameters – Meropenem 3hrs. infusion |
|||
|
Biological half-life (hrs) b[0.62-0.66 hrs] |
2.0(1.9-2.1) |
2.5(2.2-2.6) |
3.2 (2.7-3.5) |
|
Volume of distributions (L/kg) b[0.13-0.15 L/kg] |
1.05 (0.95-1.25) |
0.88 (0.82-0.95) |
0.52(0.39-0.72) |
|
Plasma clearance: (ml/min*kg) b[0.17-0,19 mL/min*kg] |
0.99(0.85-1.08). |
1.2 (0.99-1.77) |
1.95(1.62-2.40) |
|
PK/PD approach Meropenem 3hrs. infusion |
|||
|
Coverage MIC 2.0 mg/L |
11/11 |
11/11 |
20/20 |
|
Coverage MIC 4.0 mg/L |
11/11 |
11/11 |
18/20 (90%) |
|
Coverage MIC 8.0 mg/L |
6/11 (55%) |
6/11 (55%) |
6/20 (30%) |
|
Clinical outcome |
|||
|
ICU period (days) |
28 (18-42) |
36(29-44) |
40(35-45) |
|
Hospitalization (total days) |
34(26-64) |
45(42-62) |
42(37-66) |
|
Survivals (36/42) |
10/11 |
8/11 |
18/20 |
|
Nonsurvivals (6/42) |
1/11 |
3/11 |
2/20 |
Table 1 Demographic characteristics of pediatric burn patients, clinical and laboratorial data, dose regimens, PK-data, PK/PD approach (coverage based in drug serum levels), clinical outcome
Abbreviations: NAP, not applied; PK, pharmacokinetics; PK/PD, pharmacokinetics/pharmacodynamics
Notes: Microbiology data of isolates were expressed by the minimum inhibitory (CSLI) References: aBoeckh et al (2005): reference PK-data of healthy volunteers applied in vancomycin burn pediatrics study; bJaruratanasirikul & Sriwiriyajan (2003): reference PK-data of healthy volunteers 3hrs.-infusion considered in Meropenem study; cClinical Standard Laboratory Institute data base (CSLI, USA); dRybak et al (2020) for vancomycin PK/PD target recommended; eAbdul-Aziz et al.(2016) applied to Meropenem PK/PD target after 3rs-infusion.
Vancomycin therapy in pediatric septic burn patients and blood sampling
It was investigated vancomycin effectiveness with an empirical dose recommended, and if requires dose adjustment must be done soon to achieve the pharmacokinetics-pharmacodynamics target recommended by Rybak et al, in the last consensus AUCss0-24/MIC>400-600 in pediatric major burn patients.15 Then, 42 pediatric major burn patients were included in the protocol of study. Patients were allocated by age in three groups, and they received the vancomycin, empirical dose regimen recommended by 1hr.-infusion in the Set 1, as follows:
G1: 2-7 yrs (11 patients): 10-15mg/kg q6h (40-60 mg/kg daily)
G2: 8-13 yrs (11 patients): 10-15mg/kg q6h (40-60 mg/kg daily)
G3:14-18 yrs (20 patients): 10-15mg/kg q8h (30-45 mg/kg daily
Dose adjustment was done in the Set 2 if required to achieve effectiveness against Gram-positive isolated from cultures. Vancomycin coverage obtained in Set 1 was compared with results obtained in Set 2, after individualization of therapy. Blood was sampling at the 3rd and 5th hr./set of the infusion started for serum levels done by liquid chromatography. One compartment open model was chosen to investigate pharmacokinetics, and noncompartmental data analysis was applied. Drug effectiveness was evaluated by pharmacokinetic-pharmacodynamic approach, based on PK/PD target AUCss0-24/MIC>400 recommended; microbiology of Gram-positive isolates from cultures was included.
Meropenem therapy in pediatric septic burn patients and blood sampling
In addition, all pediatric major burns (42: 22M/20F) received also the recommended meropenem done by 3hrs.-infusion to attain the target (100%f∆T>MIC), MIC: minimum inhibitory concentration reported previously by Abdul-Aziz et al. for beta lactam agents largely prescribed in ICU septic patients.10
Patients allocated by age and dose regimen are described as follows:
G1: 2-7 yrs (11 patients) dose regimen 40mg/kg q8h (120 mg/kg daily)
G2: 8-13 yrs (11 patients) dose regimen 40mg/kg q8h (120 mg/kg daily)
G3:14-18 yrs (20 patients) dose regimen 20mg/kg q8h (60 mg/kg/daily eq.1g q8h)
Blood was sampling at the 3rd and 7th hr. of the infusion started for serum levels done by liquid chromatography. One compartment open model was chosen to investigate pharmacokinetics, and noncompartmental data analysis was applied. Drug effectiveness was evaluated by PK/PD approach based on target 100%f∆T>MIC and serum levels, guided also by the microbiology of Gram-negative isolates from cultures.
Analytical procedures for serum drug measurements
Drugs in blood samples were analyzed after procedure of precipitation of serum proteins with acetonitrile for vancomycin and meropenem, and purified extracts of vancomycin and meropenem were injected automatically, then analyzed by a liquid chromatograph LC10A, Shimadzu Corporation (Kyoto, Japan) as reported previously by Lopez et al. for vancomycin and by Santos et al. for meropenem.16,17 Only 0.2 mL of each sample was required for drug serum measurements, replicates n=2, using high performance liquid chromatography/ ultraviolet detection (HPLC–UV) according to the simple and accurate bioanalytical methods with adequate linearity and sensitivity. The coefficient of determination (r2>0.99) for the drug assay over the standard curve concentrations based on eight serum calibrators (C1-C8: 0.2–100 mg/L) plus the zero (C0) were acceptable; additionally internal controls were included. Calibration daily curve was accepted based on systematic error lower than 10% for the internal controls (high, medium, and low serum concentrations); then, drug serum levels in patients’ samples were determined based on the accepted daily curve using the internal standard method.
Vancomycin effectiveness based on serum monitoring
Septic major pediatric burn patients 42 (22M/20F) with preserved or augmented renal function undergoing vancomycin therapy were investigated. Vancomycin therapeutic target AUCss0-24/MIC>400 recommended by Rybak et al., at the last consensus, was considered in the study to investigate drug effectiveness by applying PK/PD target based on serum levels in pediatric major burn patients allocated by age.15 Considering the previous pharmacokinetic studies of dose adjustment done in pediatric or in adult septic burns, it is well known that its coverage is impacted by differences in pharmacokinetics of vancomycin, due a reduction on biological half-life due to increases on total body clearance age dependently, or even changes that occurs in drug elimination by vasopressors requirements were expected at the earlier stage of septic shock (Figure 1,2).18-25
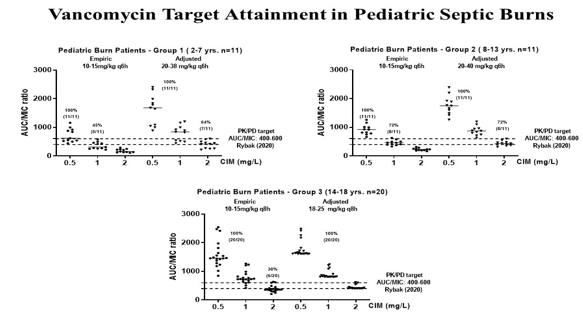
Figure 1 Vancomycin PK/PD approach for drug effectiveness after the empirical dose regimen versus individualized therapy in pediatric septic burn patients.

Figure 2 Cultures of isolated Gram-positive pathogens, Vancomycin susceptible strains isolated from pediatric septic burn patients in the combat of microbial resistance.
It was demonstrated in Set 1 that the therapeutic target was attained up to MIC 1 mg/L against Gram-positive strains, only in 59% of patients, once coverage occurred in 5/11 G1- patients and in 8/11 G2-patients receiving the same empiric dose regimen (10-15mg/kg q6h, 1hr infusion) recommended for ICU pediatric patients. Then, after dose adjustment with 20-38 mg/kg q6h regimen in Set 2, the target was attained for all patients against MIC 1 mg/L strains and extended to 64% (7/11) patients against MIC 2 mg/L after dose adjustment in Group 1-patients (2-7 years). Similarly, after drug serum measurements in Group 2-patients (8-13 years), adjusted dose regimen 20-40 mg/kg q6h was related to target achievement for all patients against MIC 1 mg/L strains and extended to 72% (8/11) patients against MIC 2 mg/L according to the data base of Clinical Standard Laboratory Institute, CSLI. In contrast, PK/PD target AUCss0-24/MIC>400 was achieved for all patients of G3-patients (14-18 years, n=20) against MIC 1 mg/L strains in Set 1 with dose regimen of 10-15mg/kg q8h, 1hr infusion, eq. 30-45 mg/kg daily; coverage was extended only in 6/20 patients against MIC 2 mg/L strains. Then, a dose regimen of 18-25 mg/kg q8h (eq. 54-75mg/kg daily) was required in Set 2 to guarantee the coverage against MIC 2 mg/L Gram-positive isolated from cultures for all Group 3-patients.
Finally, considering the initial dose regimen by 1hr infusion (Set 1) recommended, the target was attained by vancomycin up to MIC 1 mg/L against Gram-positive strains only in 55% patients receiving dose regimen recommended (10-15mg/kg q6h in G1-G2 patients (13/22); 10-15mg/kg q8h in G3-patients (6/20). While individualized therapy done in a real time, target attainment was achieved for all G1-G2 patients’ Gram-positive strains up to MIC 1mg/L isolated from cultures; it is important to highlight that the coverage against MIC 2 mg/L was extended to 15/22 G1-G2-patients and for all G3-patients. Therefore, individualization of drug therapy for vancomycin effectiveness, based on microbiology of isolates, was required up to MIC 1 mg/L for all patients, and it was extended to MIC 2 mg/L for 35/42 patients investigated. Consequently, based on this strategy, clinical and microbiological cure occurred of all patients in a short period.
Meropenem effectiveness based on serum monitoring
Pediatric major burns (42: 22M/20F) receiving meropenem and vasopressors at the earlier period of septic shock were investigated. All patients allocated by age were distributed by age in three groups. Dose regimen 40mg/kg q8h (120 mg/kg daily) done by 3 hrs.-infusion were considered for G1: 2-7 yrs (n=11) and G2: 8-13 yrs; controversially, dose regimen 20mg/kg q8h (60 mg/kg/daily) eq. 1g q8h done by 3 hrs.-infusion was based on body weight prescribed to G3:14-18 yrs (n=20) to attain the target (100%f∆T>MIC). Any significant change between groups (G1-G2, p>0,05) occurred based on meropenem coverage. Clinical and microbiological cure by meropenem occurred for all patients, once Gram-negative strains isolates up to MIC 2 mg/L were eradicated in a short period, considering MIC data of isolates from cultures in the Microbiology of central laboratory of hospital based on Clinical Standard Laboratory Institute (CSLI database, USA). Then target considered was attained by meropenem up to MIC 4mg/L against Gram-negative strains for all G1-G2 patients receiving the same empiric dose regimen (40mg/kg q8h, extended 3hr-infusion), equivalent to 120mg/kg daily. Coverage occurred for 55% for G1-G2 patients (12/22) against MIC 8 mg/L of intermediate susceptibility according to CSLI database, despite any pathogen isolated from cultures of those patients. In addition, in G3-patients, dose regimen of 20mg/kg q8h, eq. 1g q8h was prescribed based on body weight 60 (56-65) kg, med (quartiles). Meropenem coverage occurred against susceptible strains up to MIC 2 mg/L isolates for all patients, and coverage was reduced against intermediate susceptibility MIC 4 mg/L in 18/20 (90%) and MIC 8 mg/L in 6/20 (30%), despite of any Gram-negative strain isolated from these patients (Figure 3,4).
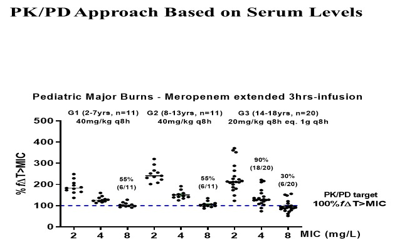
Figure 3 Meropenem PK/PD approach for drug effectiveness after the recommended dose regimen in pediatric septic burn patients age dependently.

Figure 4 ThrCultures of isolated Gram-negative pathogens, Meropenem susceptible strains isolated from pediatric septic burn patients in the combat of microbial resistance.
Pharmacokinetics meropenem studies were previously reported in septic burn pediatric and adult patients. It is well known that meropenem coverage after extended 3hrs or 4hrs infusions is impacted by pharmacokinetics alterations, because of increases on volume of distribution, with proportional prolongation of biological half-life. Additionally, vasopressors requirements at the earlier stage of septic shock can justify the increases that occurred on total body clearance in those critically ill septic patients.26-35
Outcome
Drug serum monitoring was done by blood sampling in ICU septic burn patients routinely once a week, and for patients that TDM was required to guarantee drug efficacy. Clinical cure occurred for all patients by vancomycin- meropenem combined therapy in a short period by negative cultures. Sites of infection were blood stream (51%), lungs, with pneumoniae unrelated to mechanical ventilation (17.8%), wound and bone (24.4%) and urinary tract (6.7%). Major prevalence of Gram-positive strains was related to S. aureus susceptible (MIC 0.5-1.0 mg/L) followed by Staphylococcus epidermidis (MIC 2 mg/L), and Streptococcus spp (MIC 0.25-0.5 mg/L) isolated from cultures; while it was isolated Gram-negative strains, meropenem susceptible (MIC up to 2mg/L) of Enterobacteriaceae including K. pneumoniae, and isolates of P. aeruginosa and Burkolderia cepaceae, non-Enterobacteriaceae strains of lower prevalence in the ICU of Burns. Despite of clinical cure for all patients, nonsurvivals occurred in four patients (G1-G2) and two patients from G3 based on total burn surface area higher than 60%.
None.
Authors declare that there is no conflict of interest.

©2023 Santos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.