eISSN: 2379-6367


Research Article Volume 10 Issue 3
1Clinical Pharmacokinetics Center, School of Pharmaceutical Sciences, University of Sao Paulo – Sao Paulo/SP, Brazil
2Plastic Surgery and Burns Division, Hospital das Clinicas of Medical School, University of Sao Paulo – Sao Paulo/SP, Brazil
Correspondence: Silvia R C J Santos, Clinical Pharmacokinetics Center, School of Pharmaceutical Sciences, University of Sao Paulo – Sao Paulo/SP, Brazil
Co-correspondence: Thais Vieira de Camargo, Clinical Pharmacokinetics Center, School of Pharmaceutical Sciences, University of Sao Paulo – Sao Paulo/SP, Brazil, Tel 55 11 99107-2988, Tel 55 11 95357-8930
Received: April 28, 2022 | Published: May 19, 2022
Citation: deCamargo TV, Junior EMS, Silva JM, et al. PK/PD approach to evaluate Meropenem effectiveness in critically ill burn adolescents versus young adults undergoing therapy of septic shock. Pharm Pharmacol Int J. 2022;10(3):79-85. DOI: 10.15406/ppij.2022.10.00368
Meropenem is largely prescribed to septic patients with severe infections caused by gram-negative nosocomial pathogens Enterobacteriaceae (fermenters, EB) and Non- Enterobacteriaceae (non-fermenters, NEB). Pharmacokinetics (PK) changes reported previously in burns can affect the desired outcome by physiopathology alterations during the systemic inflammatory response syndrome. The study aimed to investigate if the target is attained in septic burn patients’ adolescents versus young adults receiving the same recommended meropenem dose regimen by extended infusion. Ethical approval register CAAE 07525118.3.0000.0068 was obtained; no conflicts of interest to declare were obtained from all authors. Septic burn patients (16M/4F) were included after the fire or electrical injury (16/4), respectively. Patients have preserved renal function at admission in the Intensive Care Unit (ICU), and during the meropenem pharmacokinetic-pharmacodynamics (PK/PD) approach done by therapeutic drug monitoring (TDM) patient’s bedside number beef patients (N) was estimated according to Power & Sample Size Calculation, software v. 3.0.43; estimated power of 80% was considered. Twenty patients were allocated into two groups: G1: 10 adolescents, and G2: 10 young adults. Characteristics of burn patient’s admission were: G1/G2 16/25 yrs, 60/70 kg ideal body weight, 40/34% total burn surface area, simplified acute physiologic score III (SAPS3) 53/56 and 23/7% for the risk of death, medians. Inhalation injury occurred in 13/20 G1:G2 patients (5:8, proportion); mechanical ventilation in 18/20 (9:9; G1:G2), and vasoactive drugs were required in 15/20 patients (7:8; G1:G2) undergoing therapy of septic shock with meropenem 1 g q8h by extended 3hr infusion. Cultures were before the antimicrobial therapy started Blood was sampled and only two samples were required (2 ml/each) at the steady-state level for drug serum measurements done by liquid chromatography. Pharmacokinetics (PK) data parameters (Kel t(1/2)β, Vdss, CLT) obtained from burn patients were compared with the results reported in adults healthy volunteers. Target of 100% f∆T>MIC recommended was considered to evaluate patient’s meropenem effectiveness; biomarkers were monitored since patient’s admission and during the clinical course of septic shock During the earlier period of the septic shock, important changes occurred in the pharmacokinetics for both groups of burn patients compared with reference data considered for healthy volunteers. Additionality a significant difference between groups (G1/G2) related to the o volume of distribution (23/42 L, p=0.0310), and biological half-life (2.7/3.5 h, p=0.0035) were obtained. Blood was sampled simultaneously for meropenem serum measurements and biomarkers. Data expressed by medians were C-reactive protein 140/185 mg/L G1/G2, white blood cells 17/14 x103 cells/mm3, and neutrophils 14/12x103cells/mm3. Total isolates of gram-negate of susceptible strains Enterobacteriaceae and Non-Enterobacteriaceae from cultures (blood, alveolar bronchus lavage, wound, bone, urine) and pathogens isolated of intermediate susceptibility were investigated. Clinical cure occurred for all patients by eradicating gram-negative, susceptible, and intermediate susceptibility, considering K. pneumoniae and P. aeruginosa, MIC 4mg/L. The target of 100%f∆T>MIC was attained for all patients of both groups despite the meropenem of significant PK changes between them, and the desired outcome was reached. Finally, PK/PD approach based on drug serum monitoring done in real-time is an important tool to assess drug effectiveness in ICU septic burn patients.
Keywords: septic shock, burn patients, Meropenem 1 g q8h extended infusion, PK/PD approach, adolescents versus young adults
Major burn patients belong to the subpopulation of critically ill patients undergoing intensive care with a high risk of death, either after fire or electricity burning. These patients usually are admitted into the emergency department of tertiary hospitals with third or even fourth degree burns. High incidence of infections in major burn patients caused by nosocomial gram-negative pathogens occurs because of the severity of the trauma, high death risk, with many surgical procedures required, and prolonged hospital length of stay.1-3
Considering the destruction of the skin barrier integrity, the burn healing process, prolonged hospitalization, and the existence of immunosuppression make patients with extensive burns easily become targets of microbial and fungal colonization. Compared with postoperative surgical wounds, the incidence of infections is higher in major burns. This fact indicates that infection is one of the major complications in severe burns and the main cause of morbidity and death in these patients. Mortality associated with injury from extensive burns between 40% and 75% is related to the presence of severe infection in this critically ill patients.4-6
The most serious infections are caused by gram-negative pathogens and justify the high mortality of these patients with severe burns. It is important to note that the severity of infection increases in isolates of Klebsiella pneumonia (Enterobacteriaceae) and Pseudomonas aeruginosa (non-Enterobacariaceae) with a high frequency of development of mutant strains in these ICU patients. Therefore, when these patients in intensive care received the medium- and long-term broad-spectrum beta-lactam agents such as piperacillin and meropenem, eradication of only susceptible strains may occur, a facilitating factor in the development and mutants’ selection, which would justify the death in the ICU due to bacterial emergency.7,8
On the other hand, Carlier, et al. (2015) emphasize that so far, the serum levels of these antimicrobials have not been monitored in septic patients in Intensive Care Units, a fact that is extremely worrying due to the dire consequences for these high-risk patients during the clinical course of septic shock.9 Then, if the reduced serum level of the circulating antimicrobial is not enough to reach the desired target, therapeutic failure will inevitably occur, since the recommended empirical therapy will only promote the eradication of susceptible strains, the selection of mutants contributing to the increase of ICU deaths.10
Until now, there was no study of meropenem effectiveness, a carbapenem agent widely prescribed for the therapy of septic shock caused by gram-negative pathogens in major burns, pediatrics, or adults undergoing intensive care. More recently ABDUL-AZIZ et al. (2015, 2016) proved that the dose regimen administering 1g every 8h by prolonged infusion of 3 hours, demonstrated drug efficacy when evaluated by the PK/PD target (100%f∆T>MIC) being strongly recommended. Then, it is proposed by a controlled up to investigate major burns to study meropenem effectiveness by PK/PD approach based on serum dosage.10,15
Objective
To investigate meropenem effectiveness against hospital-acquired Gram-negative susceptible pathogens at dose regimen recommended 1 g q8h by extended 3hrs-infusion, in critically ill major burn patients, subpopulation in a controlled protocol of study by pharmacokinetic-pharmacodynamics (PK/PD) approach.
Ethical considerations: The hospital’s research ethics committee under registration CAAE 07525118.3.0000.0068 - approved the protocol by the Brazilian Regulatory Agency, CONEP (National Committee of Ethics Research). The informed consent form (ICF) from the legal representative of each patient included in the study was obtained, after being informed in detail by the medical coordinator of the research project regarding the subject of the study and the requirements for procedures to be carried out to achieve the aim of the clinical study protocol.
Casuistry: An open-label, the two-arm study for the inclusion of major septic burn patients undergoing intensive care therapy with meropenem, dose regimen of 1g q8h extended 3hrs-infusion against gram-negative susceptible strains. The protocol was carried out in the Intensive Care Burn Unit of a tertiary public hospital, Central Institute of Clinics Hospital, Medical School of the University of Sao Paulo, São Paulo, SP – Brazil.
Based on pharmacokinetics variability in major burns, the number of patients (N) was estimated according to Power & Sample Size Calculation software v.3.043 that recommended to include 9-11 patients/each arm in a total of 18-22 major burn patients for estimated power of 80%. The estimated power of 80% was related to PK-parameters that affects the coverage as follows: area under de curve/AUC, elimination rate constant (kel) and trough serum levels. Then, it was proposed to investigate 20 critically ill septic patients undergoing intensive care, of both genders, with augmented renal clearance in all of them by vasoactive drugs requirements in all of them.
Twenty patients enrolled were distributed into two groups: adolescents (teenager pediatric major burns) in Group 1 (Study group; N1=10), and major burns young adults in Group 2 (Control group; N2=10).
ICU medical nursing team initiates resuscitation procedures after volume-unresponsive hypovolemic shock in each patient needing vasoactive drugs; also performs the collection of cultures; then, the physician prescribes the systemic antimicrobial for the empirical therapy of septic shock. Patients received meropenem 1g q8h (MeronemTM) 3hs-extended infusion. Recommended empirical therapy described in the Manual prepared by the Hospital Infection Control Committee (2018-2021) was adopted regarding the starting dose regimen and the patient's renal function. Patient allocation occurred in chronological order of ICU admission. Patients of both genders, with preserved renal function, were included for comparative purposes in the meropenem effectiveness study.
After at least 48 hours of the patient’s admission to ICU, inclusion criteria were based on clinical signs and symptoms too suggestive of systemic infection characterized by leukocytosis, increased C-reactive protein, hyperthermia higher than 39ºC, hemodynamic instability requiring vasoactive drugs with indication, and the prescription of meropenem as an antimicrobial of choice for the treatment of infection caused by gram-negative strains. Cultures of fluids, secretions, and intra-operative biopsies (wound, bone) were collected for each patient before starting the therapy of septic shock. Exclusion criteria were patients with renal dysfunction, severely neutropenic (absolute neutrophil count < 500 cells/mm3), previous chemotherapy, and HIV patients with CD4 counts <200 in the last 6 months.
Patient’s renal function based on creatinine clearance, considering serum creatinine, age, and gender was according to data from Fleury Medicine and Health – Central laboratory certified in the hospital. Results from cultures of blood, urine, tracheal bronchus lavage, and intraoperative biopsies of wound and bone were analyzed in Microbiology of Central Laboratory of the hospital, and MIC dataset, certified by Clinical Laboratory Standard Institute (CLSI).
Blood sampling for meropenem serum monitoring: Blood samples were collected after meropenem therapy for at least 48 hours to ensure steady-state serum levels were reached. Then, the sequential blood collection of two samples (2 mL each/gel tube) from the central venous catheter was performed by the nursing-physician team on duty. The first collection was done at the end of extended infusion (3rd hr), and the second two hours afterward (5th hr). Samples were kept under refrigeration during blood sampling up to the transport to the laboratory. After centrifugation (2800 g for 20 minutes) the serum was obtained, properly identified, and stored in a refrigerator for drug analysis on the same day or kept in a deep freezer until the analysis was performed.
Meropenem serum dosage: Quantification of the analyte was performed by high-performance liquid chromatography developed and validated in the laboratory of Clinical Pharmacokinetics Center, based on the standard operating procedure of our laboratory, internal standard method reported by Santos et al, by RDC 205/2017 - ANVISA, Brazilian Regulatory Agency.12
PK-investigation study and PK/PD approach
PK-parameters and the equations applied to investigate the pharmacokinetics of meropenem, based on serum levels were described as follows:
|
Parameter |
Description |
Equation |
|
Infusion rate |
k0 (mg/h) |
Dose/Tinfusion |
|
Through levels at steady-statee |
Cssmin(mg/L) |
Estimated data (C=C0*e–kel*T) |
|
Serum levels obtained after dose infusion (3rd-5th hr.) |
Css (mg/L) |
Obtained data related to C1 and C2 |
|
Elimination rate Constant |
kel (h–1) |
(LN(C1) –LN(C2) /(T2–T1) |
|
Biological half-life |
t(1/2)b(h) |
0.693/ Kel |
|
The area under the curve |
ASCtss (mg · h/L) |
Trapezoidal rule (time dose interval: t) |
|
Total body clearance |
CLT (L/h_ mL/min*kg) |
Dose/ASCtss |
|
Volume of distribution |
Vdss (L_L/kg) |
CLT/kel or Dose/ (kel. ASCtss) |
Abbreviations: t, time dose interval; Css, serum levels at the steady-statee; T, time. Ref.: Dipiro et al.13
It is important to highlight that the pharmacokinetic data obtained for meropenem in major burns were compared to reference values reported in a study conducted on healthy volunteers receiving the same dose regimen by extended infusion.14
In addition, PK data permitted to estimate the meropenem coverage by the correlation of pharmacokinetics (in vivo) with the pharmacodynamics (in vitro measurements) related to the meropenem susceptibility, expressed by the minimum inhibitory bactericidal concentration of pathogen isolated from each patient investigated.
Since the effectiveness of the carbapenem agent is time-dependent, the estimation of the predictive index of drug effectiveness was based on pharmacokinetics (serum trough levels, elimination rate constant) and on pharmacodynamics, related to the minimum inhibitory bactericidal concentration, (MIC90) able for eradicating 90% of the colonies isolated from the cultures. Thus, the predicting index for meropenem effectiveness is estimated to assess the coverage of antimicrobial therapy against pathogens isolated from major burn patients. Drug effectiveness related to meropenem serum trough levels, the free fraction that remains above the minimum bactericidal inhibitory concentration of the antimicrobial against the isolated pathogen, percentage of the time dose interval (%fΔT>MIC): f (free drug level): is the free fraction of meropenem in circulating blood; ΔT: time dose interval between two consecutive doses; MIC: minimum bactericidal inhibitory concentration for 90% of culture colonies.
Minimum bactericidal inhibitory concentration was determined; it is applied in the Microbiology of the Central Laboratory of the hospital to investigate the MIC database recommended by the Clinical & Laboratory Standard Institute (CLSI). It is noteworthy that the PK/PD target of 100%fΔT>MIC described was considered in this study to investigate if the target is attained in major burned patient’s subpopulation investigated in the present controlled clinical protocol.10
According to Power & Sample Size Calculation, software v. 3.0.43, it is proposed in the protocol to investigate 9-11/each arm in a total of 18-22 major burn patients; estimated power of 80% was related to PK-parameters that affect the coverage as follows: area under de curve (AUC), elimination rate constant (kel) and trough serum levels. The controlled study protocol included 20 patients classified with major burns, distributed in two groups of 10 patients each, based on age: adolescents (G1) and young adults (G2), who received recommended therapy for septic shock with meropenem 1 g q8h, by 3hrs-extended infusion. The demographic and ICU admission characteristics, and clinical and laboratory data of the investigated patients were described based on population data (Table 1).
Patients |
Group 1 n=10 |
Group 2 n=10 |
Statistics |
(N=20) |
Teenagers |
Young adults |
|
Demographic data |
|||
Gender |
9M/1F |
7M/3F |
(7/3)0.5820a |
Age (yrs) |
16(12-17) |
25(24-27) |
0.0002b |
IBW (kg) |
63 (55-67) |
71 (55-75) |
0.2392b |
Height (cm) |
165 (156-167) |
171(161-175) |
0.0727b |
BMI (m2) |
1.66 (1.62 – 1.72) |
1.83 (1.66 – 1.91) |
|
BSA (kg/m2) |
24 (22 - 25) |
24 (22 - 24) |
0.8787b |
Admission data |
|||
TBSA higher than 40% |
40 (27-58) |
34 (31-38) |
0.4722b |
SAPS3 |
53 (48-59) |
56 (39-60) |
0.9094b |
Death risk factor |
23 (16-33) |
7 (6 -11) |
0.0016b |
Inhalation injury (proportion) |
(5/10) |
(8/10) |
(13/7) 0.3498a |
Thermal injury (proportion) |
(7/10) |
(9/1) |
(16/4) 0.5820a |
Electrical injury (proportion) |
(3/10) |
(1/9) |
(4/16) 0.5820a |
Mechanical ventilation (proportion) |
(9/1) |
(9/1) |
(18/2) 1.0000a |
Vasoactive drug requirement/weaning (proportion) |
(7/3) |
(8/2) |
(15/5) 1.0000a |
Biomarkers at admission |
|||
C- reactive protein (mg/L) |
260 (184-289) |
146 (89-213) |
0.0637b |
Leucocytes (mil cel./mm3) |
17.13 (14.19-19.16) |
12.85 (10.25-17.59) |
0.3442b |
Neutrophiles (mil cel./mm3) |
13.37 (10.57-15.68) |
11.09 (7.97-15.22) |
0.3841b |
Serum creatinine (mL/min) |
170 (153 - 182) |
145 (126-158) |
0.1806b |
Creatinine clearance (mL/min) |
174 (170 - 194) |
180 (142-207) |
1.0000b |
Biomarkers at TDM |
|||
C- reactive protein (mg/L) |
140 (97-325) |
185 (127-246) |
0.7620b |
Leucocytes (mil cel./mm3) |
16.69 (14.09-21.00) |
14.31(11.41-20.48) |
0.6772b |
Neutrophiles (mil cel./mm3) |
13.76 (10.57-18.54) |
11.63 (9.36 – 18.30) |
0.9096b |
Serum creatinine (mL/min) |
119(60-195) |
122 (87-181) |
0.9698b |
Creatinine clearance (mL/min) |
135(66-212) |
190 (92-204) |
0.7052b |
Pharmacokinetics |
|||
Biological half-life (hours) |
2.7 (2.5-3.2) |
3.5 (3.2-4.2) |
0.0035b |
Apparent volume of distribution (L) |
23 (22-37) |
42 (34-58) |
0.0310b |
Total body clearance (L/h) |
6.3 (5.8-8.3) |
8.4 (7.2-9.0) |
0.2161b |
PK/PD approach |
|||
Coverage up to MIC 4mg/L |
136 (131-140) |
147 (137-163) |
0.0633b |
Clinical cure |
9/10 |
9/10 |
1.0000a |
Microbiological cure |
9/10 |
9/10 |
1.0000a |
Clinical outcome |
|||
ICU period (days) |
55 (26-67) |
64 (27 – 63) |
0.7050b |
Hospitalization (total days) |
36 (29-44) |
42 (39-60) |
0.8203b |
Survivals |
9/10 |
9/10 |
1.0000a |
Nonsurvivals |
1/10 |
1/10 |
1.0000a |
Table 1 Demographic characteristics-Clinical and Laboratorial data of patients investigated
Abbreviations: M, male; F, female; SAPS3, simplified acute physiological score III; TBSA, total burn surface area; TDM, therapeutic drug monitoring. Statistics – afishers Contingency Test (proportion); bMann Whitney, significance p<0.05. Graph Pad Prism 7.0 (Graph Pad Instat software).
All demographic data from the two groups of patients investigated when compared between groups were comparable, except related to age, which showed a significant difference between the groups. The admission profile of patients related to the total burned surface area (TBSA), SAPS3 (Simplified Acute Physiology Score III) and risk of death, type of thermal or electrical injury are described through population data and comparison between groups, Mann Whitney's nonparametric test, and Fisher's contingency test.
There was no significant difference between groups regarding ICU admission data, except for the risk of death associated with the SAPS 3 admission severity score. The laboratory profile related to inflammatory biomarkers and renal function were performed in the daily laboratory routine of patients in the hospital's ICUs. The results refer to the serum dosage of inflammatory biomarkers, such as C-reactive protein, the white blood cell count – leukocytes, and neutrophils of the daily hemogram; creatinine clearance was estimated based on the serum creatinine, an endogenous marker of glomerular filtration rate.
Daily dose and dose regimen recommended in the septic shock therapy of patients classified as major burns were expressed, in both groups, as daily dose of regimen 1 g q8h, through an extended infusion of three hours, normalized to ideal body weight. There was no significant difference between groups in terms of the daily dose, dose regimen, area under the curve, and trough level.
Meropenem effectiveness, based on the index of drug effectiveness expressed as %f∆T>MIC was investigated in both groups after the recommended dose regimen of 1 g q8g via an extended three-hour infusion. It was considered to reach the recommended PK/PD target of 100%f∆T>MIC. The individual data obtained from the two groups of patients investigated were evaluated in terms of effectiveness, expressed as the percentage of target achieved, Percentage of Target Attained (PTA) illustrated in Figure 1.
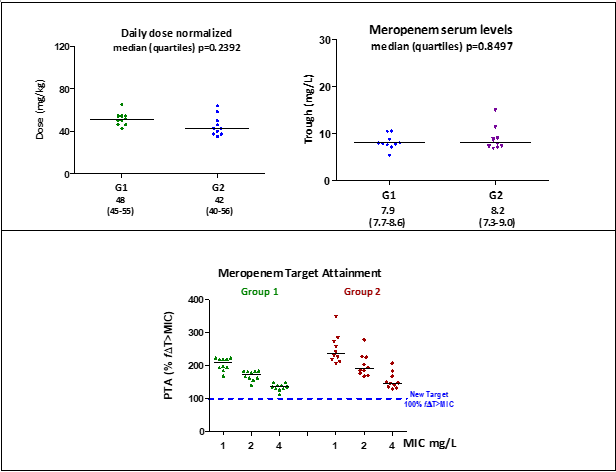
Figure 1 Daily dose, serum trough, and meropenem coverage: meropenem coverage in investigated patient populations – dose 1 g q8h, extended 3hrs-infusion.
Abbreviations: Target PK/PD 100%f T>CIM. Statistics: medians (interquartile), Mann Whitney Prism v.5.0; significance p<0.05. Source: PK/PD target recommended Abdul-Aziz et al. (2016).
Meropenem coverage against gram-negative susceptible strains (CIM < 2mg/L) was guaranteed according to the CSLI database. In addition, meropenem coverage was increased against strains of intermediate susceptibility MIC 4 mg/L for patients of both groups of patients investigated.
Sites of infection occurred in the circulatory blood stream, the genitourinary tract, and in lungs. The total of isolates was 25 pathogens, which were stratified into Gram-positive and gram-negative strains. Among the Gram-positive pathogens, Staphylococcus spp (7/25) and Enterococcus faecalis (3/25) were isolates; then, in these patients, vancomycin 1g q12h 1hr infusion was combined with meropenem therapy, with eradication of all Gram- positive strains with cure of infection in a period of 7 to 10 days of therapy.
In addition, among the Gram-negative pathogens, 5/25 isolates of Enterobacteriaceae (Enterobacter cloacae. Klebsiella pneumonia. Proteus mirabilis) were registered all susceptible to meropenem (MIC 0.25 mg/L). High incidence of isolates was related to non-Enterobacteriaceae (10/25) described as follows: Pseudomonas aeruginosa susceptible strain (MIC 0.25 mg/L) strain in one G1-patient, and isolates of intermediate susceptibility in three G2-patients, MIC 4 mg/L. It was recorded six isolates of Acinetobacter baumannii, colistin susceptible strains (MIC 0.5 mg/L). Then, combined therapy of meropenem and colistin was adopted in those patients. It registered 18 survivals, and only two deaths, one death in G1 patients (TBSA 75%) and another death in G2-patient (TBSA 45%), despite clinical cure reached up to seven days of antimicrobial therapy.
Changes in PK of hydrophilic antimicrobials in ICUs have been extensively discussed in the past, but there is still no consensus regarding the high pharmacokinetic variability reported in several subpopulations of critically septic patients in studies reported previously. Unfortunately, meropenem serum monitoring and other beta-lactam agents as well are not usually performed in tertiary hospitals prescribed for the therapy of septic shock. Consequently, drug serum measurement of these agents is not routinely done for septic patients undergoing intensive care in hospitals. Then currently, septic shock therapy is guided in hospitals only by clinical course, cultures data, C-reactive protein, and the absolute count of leukocytes and neutrophils, which is still not enough for decision-making to allow early intervention in medical management to reach the desired clinical outcome.
More recently, was reported especially in the early phase of septic shock that PK- changes that occur in patients undergoing intensive care for septic shock can influence the coverage of beta-lactam agents. Considering meropenem, a carbapenem agent with high penetration into lung tissue and soft tissues, it is noteworthy that the PK/PD target initially recommended by Ikawa et al. (2008) was 40%fΔT>MIC. A target of 60%fΔT>MIC was suggested by Silva Junior (2017); and finally, a new target of 100%fΔT>MIC that guaranteed the cure of septic shock by gram-negative strains was recommended by Abdull-Aziz (2016).15-17
It is important to note that, in the last two decades, an increasing selection of mutants has emerged by eradicating only susceptible strains (MIC 0.25–2 mg/L) for Gram-negative pathogens such as K. pneumonia, P. aeruginosa, and A baumannii, which are highly prevalent in patients in most ICUs. More recently, Abdull-Aziz (2015) highlighted the importance of maintaining the highest serum trough levels for meropenem at the time dose interval, to prevent the development of mutant strains, thus combating bacterial emergence. The circulating concentration of the antimicrobial equivalent to the minimum inhibitory concentration (MIC) eradicates only the susceptible strains of MIC 0.25 up to 2 mg/L. According to the same authors, there is a range of meropenem concentrations called mutant selection window (MSW) that is related to the development, growth, and selection of mutant strains. Therefore, meropenem serum levels should always exceed the upper limit of this window, allowing its serum levels to reach a circulating concentration that prevents development, thus promoting the eradication of mutant strains. This concentration, equivalent to the upper limit of the window (MSW), was called the author’s mutant prevention concentration (MPC).10
Thus, new strategies have been suggested to make the dose of 1 g q8h effective for meropenem in the treatment of infections caused by susceptible Gram-negative nosocomial pathogens. Several controlled protocols were conducted with meropenem in critically ill patients undergoing intensive care through strategies related to the duration of the infusion instead of the intermittent 0.5 h infusion recommended initially in the package insert by the manufacturer. Consequently, which target must be reached to guarantee the antimicrobial coverage against Gram-negative intermediate susceptibility strains with MIC 4-8 mg/L?.17
In any case, there is already a consensus in the literature that after the intermittent infusion of 0.5 h, the meropenem coverage against Gram-negative pathogens is guaranteed only up to MIC 2 mg/L for all targets considered previously.17 Therefore, the target of 100%fΔT>MIC recommended by Abdull-Aziz (2016) can support attention during septic shock therapy against mutant pathogens of intermediate susceptibility, MIC 4-8mg/L.16
A study of the superiority of coverage for meropenem was conducted by Silva Junior (2017) including 20 septic adult patients with major burns, who received meropenem 1 g q8h by intermittent infusion of 0.5 h (n=10) in one group, and an extended 3 hrs-infusion in another group of 10 patients. It was demonstrated by the authors a clear improvement in the clinical outcome with the extended infusion since the coverage was guaranteed up to MIC 8 mg/L for all patients. However, a target of 60%fΔT>MIC was considered by these authors to measure the effectiveness achieved, since the intermittent infusion provides coverage up to MIC 2 mg/L; a coverage reduction was reported for isolates of intermediate susceptibility, MIC 4 mg/L (8/10), and MIC 8 mg/L (4/10) patients.17
Another prospective clinical protocol was performed in burn septic patients, adults versus pediatrics (teenagers) who received 1g q8h by 3hrs-extended infusion to investigate meropenem effectiveness. The authors found a significant difference between groups in terms of volume of distribution and biological half-life, with total body clearance remaining unchanged. It is important to highlight that those pediatric patients had earlier weaned from mechanical ventilation and vasoactive drugs, compared to burn young adults. Clinical outcome was achieved by all patients at the PK/PD target considered. Clinical and microbiological cure against pathogens up to MIC 4 mg/L were achieved for all patients, despite coverage, decreased by 50% (7/14) of patients investigated against gram-negative strains MIC 8 mg/L.18
More recently, it was reported recently by Messiano et al. (2022), in a clinical protocol that was conducted in major burn adult septic patients (TBSA>35%) and preserved renal function, who received vasoactive drugs only at the early phase of septic shock. The authors reported pharmacokinetic data in three sets of meropenem TDM PK changes that affect meropenem coverage were described only in the early period of septic shock from initiation of therapy (48-72 hours), compared to the late period of septic shock, periods from day 10 to day 15 periods. It was described as a pronounced increase in the volume of distribution and a prolongation of the biological half-life affecting meropenem coverage against the isolates up to MIC 8 mg/L, including P. aeruginosa and K. pneumoniae, only in the early stage of the septic shock. On the other hand, it is important to highlight that, between the 10th and 15th days of treatment (late period of systemic inflammatory response syndrome (SIRS) the coverage was guaranteed against isolates only up to MIC 2 mg/L. Based on the results reported by the authors, it is relevant to consider that septic patients with a preserved renal function who generally receive vasoactive drugs in the early phase of shock during the SIRS have shown profound changes after the extended infusion, positively impacting the meropenem coverage in the critical period of the nosocomial infection.19
In another study, Kupa et al. (2019) reported changes in pharmacokinetics that affected pharmacodynamics in 13 critically ill burn patients (SCTQ 13-38%) after thermal or electrical injury, during meropenem treating septic shock. These patients had preserved renal function, with vasoactive drugs requirements. A pronounced increase in the volume of distribution with a proportional prolongation of the biological half-life of meropenem was recorded in these patients during SIRS, at the early period of septic shock. However, total body clearance was reduced by 50%. This fact was to be probably due to the reduction of expression in drug transporters OAT3 and MRP4, involved in the renal tubular secretion of beta-lactam agents.25 All patients reached the therapeutic target considered 100%fΔT>MIC after 3hrs-extended infusion against pathogens of intermediate susceptibility, MIC 4mg/L. So, regardless of the type of intermittent or extended infusion of meropenem 1 g q8h dose regimen, if serum levels are lower than required bactericidal in the circulatory stream, soft tissue, and bone, bacterial resistance inevitably will be developed in these patients.16,20,21
Series of pharmacokinetic studies with meropenem were performed previously in critically unburned patients, after 3hrs-extended infusion. Unfortunately, lower targets were considered (40% and 50%fΔT>MIC) that permitted mutant selection and an increase in deaths in ICU.22-24 All changes in pharmacokinetics pointed out in critical septic patients were compared with the data reported in healthy volunteers.14
It is important to highlight that, in general, we found a concordance between the data obtained in this study and the studies previously reported regarding the change in pharmacokinetics that occurs in different proportions in critically ill patients with preserved renal function, after intermittent infusion or extended infusion. It is also noteworthy that, after the extended 3hrs-infusion, increases the volume of distribution during SIRS with a proportional prolongation of the biological half-life only at the early stage of septic shock.
Regarding the coverage of meropenem and the target achieved by the carbapenem, based on the target of 100%fΔT>MIC against the isolates up to MIC 4 mg/L, the superiority of the extended infusion over the intermittent infusion, 0.5 hours, as evidenced, addressed in most of the works discussed in this review comparing with our data in the present study.25
Clinical management for ICU septic patients is based on cultures; the initial therapy is based on the empirical prescription of antimicrobials at the recommended dose at the onset of septic shock. Also, the renal function of each patient must be considered; the clinical course of therapy for these patients today is guided by daily clinical evolution, the routine of C-reactive, and isolated cultures data, when available in time on the network.
In fact, the cost-benefit of antimicrobial serum dosage justifies the laboratory support done in real-time for the medical team, providing clinical intervention based on meropenem coverage by applying the tool of the PK/PD approach. It is also noteworthy that such a measure implemented in the routine of the hospital's central laboratory would prevent the development of mutant strains, especially for K. pneumonia, among the Enterobacteriaceae, and for P. aeruginosa, non-Enterobacteriaceae, because of the sub-therapy that occurs during systemic inflammatory response syndrome (SIRS), especially in the early period of septic shock in patients with vasoactive drugs requirements. Finally, meropenem serum monitoring and PK/PD approach done in real-time must be included in the therapy of septic shock pack to reduce mortality in ICUs.
None.
Authors declare that there is no conflict of interest.

©2022 deCamargo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.