eISSN: 2379-6367


Review Article Volume 6 Issue 6
1Department of Pharmaceutical Sciences, Kumaun University Bhimtal Campus, India
2School of Pharmacy, BBD University, India
Correspondence: Rajiv Gupta, School of Pharmacy, BBD University, Lucknow 226028 (U.P.), India , Tel 91-9839278227
Received: October 26, 2018 | Published: November 19, 2018
Citation: Sharma S, Kaur R, Upadhyaya K, et al. An overview of pleiotropic effect of statins in cardiovascular disease. Pharm Pharmacol Int J. 2018;6(6):435-439. DOI: 10.15406/ppij.2018.06.00214
HMG CoA-reductase (3-hydroxy-3-methylglutaryl coenzyme A reductase) inhibitors, also called statins, are at present exerting an effect on the therapeutic treatment decision for hypercholesterolemia. Hypercholesterolemia is a known risk factor for cardiovascular malady, and statin treatment has prompted a significant decline in grimness and mortality from undesirable cardiovascular events, stroke, and fringe blood vessel malady. Notwithstanding accomplishing a remedial diminishing in serum cholesterol levels, statin treatment appears to advance different impacts that are autonomous of changes in serum cholesterol. These "pleiotropic" impacts incorporate constriction of vascular aggravation, enhanced endothelial cell work, adjustment of atherosclerotic plaque, diminished vascular smooth muscle cell relocation and expansion, and restraint of platelet total. This review an attempt to compile published reports on the pleiotropic impacts of statins at the cellular level.
Keywords: statins, pleiotropic effects
Statins also known as 3-hydroxy-3-methylglutaryl co-protein A (HMG-CoA) reductase inhibitors. These are the medications regularly utilized to lower down the cholesterol and appeared to decrease the frequency of essential and optional coronary illness in clinical trials.1–3 They were found in 1970s. Statins are competitive inhibitors of hydroxylmethyl glutaryl coenzyme-A reductase (HMGCR), which is the rate limiting enzyme in cholesterol biosynthesis.4,5 The positive results of statin treatment in experimental animals were confirmed and reached out to patients in extensive scale clinical investigations that built up a strong influence of statin in the treatment associated with fundamentally lessened,rates of mortality in patients with atherosclerosis.4,6,7 Nevertheless evoking helpful impacts in the cardiovascular framework that incorporates enhanced endothelial function furthermore, neo-angiogenesis, HMG COA inhibitors apply pleiotropic activities in different tissues including the focal apprehensive framework. For example, statins seem to apply mitigating activities in illnesses, for example, rheumatoid joint inflammation, Alzheimer and Parkinson's ailment, and various sclerosis.8
Statins pleiotropy: associated with lowering of cholesterol
Statins are believed to have “pleiotropic effects”, which is a term referred as the useful impacts of statins which are not dependent on levels of cholesterol (Figure 1).8,9 According to these pleiotropic impacts, positive results utilizing statin treatment have been acknowledged in cardiovascular disease (CVD) and in addition other ailment states including disease, sepsis, dementia, immune system ailment, macular degeneration, osteoporosis, and incendiary gut infection.8‒15 The water solvency of statins may decide some of their pleiotropic impacts and is straightforwardly inferable from their chemical structure.16 As 60-70% of serum cholesterol is synthesised from liver and HMG-CoA reductase is the vital, rate-constraining compound in the cholesterol biosynthetic pathway, hindrance of this enzyme by statins brings about an dramatic lessening in low density lipoprotein (LDL)-cholesterol (Figure 2). Likewise, lessening of LDL-cholesterol prompts upregulation of the LDL receptor and expanded LDL leeway. The downfall in serum cholesterol levels is in this manner thought to be the essential component fundamental the helpful advantages of statin treatment in cardiovascular sickness.17,18
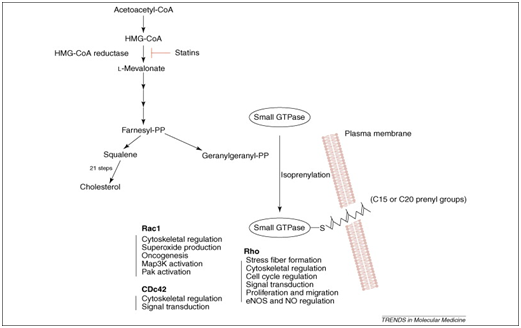
Figure 2 Isoprenoids and statins: cholesterol biosynthesis pathway and the impacts of HMG-CoA-reductase hindrance by statins.
Statins exerts their pleiotropic effects by hindering the conversion of HMG-CoA to L-mevalonic corrosive, statins keep the union of imperative isoprenoids, for example, farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP), which are antecedents of cholesterol biosynthesis (Figure 1).19 These intermediates fill in as imperative lipid connections for the post-translational change of proteins, for example, nuclear lamins, Ras, Rho, Rac and Rap.20 Protein isoprenylation empowers appropriate subcellular confinement and trafficking of intracellular proteins. Given that isoprenylated proteins might control various cell capacities, it is not amazing that statins may have extra impacts past lipid lowering. In fact, late examinations propose that statins may be included in immunomodulation, neuroprotection and cell senescence.21‒26 Rho is the real focus of GGPP; in this way, restraint of Rho and its downstream target, Rho kinase is a probable system intervening a portion of the pleiotropic impacts of statins on the vascular membrane.27,28,31 Rho kinase increase the affectability of vascular smooth muscle to calcium in hypertension, and coronary spasm. Conversely, activation of Rac prompts the development of lamellipodia, membrane unsettles, and oxidative anxiety, while initiation of Cdc42 initiates actin-rich surface projections called filopodia.29‒31
A study known as “Framingham Heart study” has shown that hoisted cholesterol is a vital risk factor for cardiovascular illnesses and lower cholesterol levels are related with lower cardiovascular dangers.24 Late proof likewise demonstrates that more escalated bringing down of LDL- cholesterol by statins is related with more prominent clinical advantages.25 The systems credited to lipid bringing down with statin treatment incorporate atheromatous plaque adjustment, change of the atherosclerosis movement and enhanced endothelial capacities.26 Consequently, statins diminish cardiovascular occasions in hypercholesterolemic as well as normocholesterolemic patients with coronary illness (CHD) or cardiovascular risks.17
Atherosclerotic plaque and endothelial injury
Atherosclerosis is responsible for the continual process of endothelial injury and dysfunction with subsequent remodeling and repair.32 After endothelial damage happens, platelets are among the first to react by clinging to the damage site and selecting macrophages to the range of damage.33,34 Macrophages phagocytize lipid-loaded platelets and move into the intima where they take in more lipids. These macrophages are commonly alluded to as ''froth cells,'' as the substantial measure of lipid they ingest gives them a frothy appearance when seen minutely.35 Furthermore, VSMCs move from the media to the intima close to the territory of endothelial damage with a large portion of them taking in a lot of lipid to likewise move toward becoming froth cells. Serum lipoproteins amass in the intima at these territories of damage and alongside these three cell sorts, turn out to be a piece of what is named the ''greasy streak''-the most punctual sore in the arrangement of atherosclerotic plaque.33
Fatty streak is the first visible lesion in the process of atherosclerosis. It appears as an irregular yellow-white discoloration on the luminal surface of an artery. They can be visualized without magnification. The fatty streak experiences additionally renovating to frame a sinewy top made for the most part out of VSMCs and the extracellular lattice they emit. Hidden this top is a center of necrotic cell flotsam and jetsam and froth cells pressed with lipid.35 Interruption of the sinewy top starts a provocative reaction that can prompt thrombus arrangement, with possible joining into the complex atherosclerotic plaque itself.33 The final product of this procedure is narrowing or finish blockage of the vascular lumen with a resultant trade off in blood stream. A systematic assessment of fatty streak formation carried out in fetal aortas from normocholesterolemic mothers, hypercholesterolemic mothers and mothers who were hypercholesterolemic only during pregnancy suggests that intimal LDL accumulation and oxidation contributes to monocyte recruitment in vivo.33
Neointimal hyperplasia associated blood vessel wall injury
Neointimal hyperplasia is a noteworthy reason for reconstructive disappointment in patients after angioplasty, stenting, and sidesteps procedures.36 Both open and endovascular strategies create blood vessel harm that triggers a physiologic reaction.36 Platelets quickly cause thrombus development at the surface of vessel divider damage.36, 37 An incendiary course follows, with monocytes, neutrophils, and lymphocytes moving to the region of intimal damage.35 Average VSMCs move to this region of intimal damage too, and throughout the following a while they multiply and deliver a lot of extracellular framework.38‒40 The entirety of these cell and sub-atomic reactions prompts neointimal hyperplasia and restenosis of the instrumented vein.40
There are some main targets of statins like Isoprenylated Proteins, Rho/Rho Kinase, Rac and Peroxisome Proliferator–Activated Receptor
Statins: isoprenylated proteins
By restraining mevalonic acid biosynthesis, HMG COAR Inhibitors keep the blend of isoprenoid intermediates farnesylpyrophosphate and geranylgeranylpyrophosphate.41 FPP and GGPP fill in as lipid connections for the post-translational modification of heterotrimeric G proteins, including Ras and Rho.42 Ras and Rho control cell expansion, separation, apoptosis, and the cytoskeleton.43 In endothelial cells (ECs), Ras translocation is subject to farnesylation, while Rho translocation is reliant on geranylgeranylation.44,45 Although the restraint of isoprenoid moderate amalgamation is vital to the conceivable pleiotropic impacts of statins, it is un-clear whether the essential LDL-C–lowering advantage of statins is a direct result of diminished cholesterol generation and lessened mevalonic corrosive creation or upregulation of the LDL receptor.46 There is a relative scarcity of human examinations on the levels of FPP and GGPP with perpetual statin treatment.
Statins: Rho/Rho kinase
The organic impacts of Rho are interceded by its downstream effectors, including ROCK, protein kinase N-related kinases, citron kinase, rhotekin, mDia and the myosin restricting subunit of myosin light-chain (MLC) phosphatase.47 Rho kinases (ROCKs) are protein serine/threonine kinases of 160 kDa that add to the downstream impacts of Rho GTPases. ROCK movements to a dynamic open adaptation when RhoA ties to ROCK.48 ROCKs control actin cytoskeletal changes through impacts on myosin light chain phosphorylation. This influences central attachment complex arrangement, smooth muscle withdrawal, cell relocation, and quality expression.49 ROCK activity is frequently hoisted in disorders of the cardiovascular framework.50 Accordingly, statins could influence vascular smooth-muscle compression in any event somewhat through impacts on Rho/ROCK.51,52 Through hindrance of isoprenylation of Rho, translocation of Rho to the cell layer is hindered and the downstream initiation of ROCK is diminished.53 Without a doubt, ROCK inhibitors anticipate cerebral vasospasm after subarachnoid discharge,54 hinder the advancement of atherosclerosis55 and anticipate blood vessel renovating after vascular damage.56
The Rho/ROCK pathway could likewise control cell works other than the actin cytoskeleton. For instance, ROCK can phosphorylate insulin receptor substrate-1 (IRS-1) and adjust the insulin/PI3K/Akt pathway. The Rho/ROCK pathway is included in oxidative push, aortic firmness and changes in circulatory strain. Besides, ROCK controls cell survival through phosphorylation of the protein kinase B/Akt and FOXO.57‒59 ROCK can likewise control adipogenesis and myogenesis. In p190-B Rho GAP-insufficient mice, the Rho/ROCK pathway is actuated constantly and there is a deformity in adipogenesis with a preference towards myogenesis. Different procedures or conditions including the RhoA/ROCK pathway incorporate angiogenesis, hypertension, cardiovascular hypertrophy, perivasclar fibrosis and aspiratory hypertension. Fasudil, a particular ROCK inhibitor, enhances endothelial capacity in patients with coronary conduit ailment.60‒64 These discoveries propose that ROCK restraint may add to a portion of the pleiotropic impacts of statin treatment.
Statins: RAC
Two vital effector-reaction pathways lie downstream of Rac: cytoskeletal redesigning furthermore, responsive oxygen species (ROS) era. Rac1 impacts different cytoskeletal rebuilding proteins, for example, Wiskott-Aldrich disorder protein, calmodulin-restricting GTPaseactivating proteins and p21-actuated kinase. Rac1 likewise ties to p67phox and prompts enactment of the NADPH oxidase framework and consequent era of ROS. In fact, Rac movement is firmly identified with ROS creation and ROS produced by NADPH oxidase in light of development factors and fiery cytokines is interceded by Rac.65 Imperatively, statins hinder Rac1-intervened NADPH oxidase movement and in this manner lessen angiotensin II-actuated ROS generation and hypertrophy in smooth muscle and heart.66,67 The initiation of Rac1 in the vascular divider has been related with atherosclerosis, neointimal multiplication, cardiovascular hypertrophy and endothelial brokenness.68 Rac1 has numerous parts in various cell forms and cardiovascular physiology.69 Consequently, Rac1 hindrance may likewise add to a portion of the pleiotropic impacts of statins.
Statins: peroxisome proliferator-activated receptor
Statins have been appeared to enact peroxisome proliferatorenacted receptors (PPARs).70 Statins intensely diminish lipopolysaccharide-related aggravation in wild-sort mice yet not in PPARα-invalid mice, autonomous of cholesterol-bringing down mechanisms.71 Statins increment PPAR-γ action and repress lipopolysaccharide instigated tumor putrefaction factor-α and monocyte chemotactic protein-1 activity.72,73 The organization of simvastatin in mix with PPAR-γ agonists inspires added substance gainful vascular effects.74 Atorvastatin diminishes progressed glycation finished results in rats and lessens fibroblast multiplication and heart fibrosis, which was switched with the PPAR-γ adversary GW9662.72 Statins diminished ROS creation by expanding the mRNA articulation of the PPAR-γ coactivator, which is an essential controller of mitochondrial biogenesis. However, statins, particularly the high-power statins, increment the danger of diabetes mellitus. Thus, the capacity of the PPAR-γ agonists, thiazolidinediones, to bring down blood sugar is rather than the impacts of statins on PPAR-γ and exhibits the unpredictable idea of statin communications with different pathways, including glucose digestion.
Statins: vascular smooth muscle
The expansion of vascular SMCs is critical in vascular injury pathogenesis.74 Transplant arteriosclerosis is an insusceptible reaction coordinated against giver ECs and vascular SMCs free of hypercholesteremia that is as yet constricted by statins.75 Inhibition of isoprenoid blend by statins diminished platelet-determined development factor–induced DNA blend in vascular SMCs by expanding the cyclindependent kinase, p27Kip1, which was potentially intervened by Rho GTPase.76 Simvastatin diminishes intimal thickening and lessens cell expansion, leukocyte aggregation, and platelet-determined development consider receptor phosophorylation LDL receptor–deficient mice.77 In vitro, atorvastatin decreases the impacts of the proinflammatory cytokine IL-18, which hinders SMC movement, atomic factor-κB initiation, and framework metalloproteinase-9 expression. In ox-like aspiratory supply route SMCs, atorvastatin represses the relocation of pneumonic supply route SMC, which was turned around by GGPP and mevalonate, again ensnaring the potential for the Rho/ROCK pathway in SMC proliferation.78,79
Statins and platelet function
In (ACS) Acute Coronary Syndromes, a major factor, which plays a critical role, is platelets, which are allied in the formation of mural thrombus at the site of vascular injury and plaque rupture. This concept is directly linked with the enhanced cholesterol: phospholipid ratio in platelets.31 HMG COA reductase inhibitors or Statins affect the functions of platelets, but there is no well understandable mechanism for this concept. According to well considered effects of endothelial NO, inhibits the platelet aggregation. Statins upregulates the endothelial nitric oxide, which considerably decreases the reactivity of platelets. Further mechanism shows the reduced production thromboxane A2 and modifies the platelet membrane cholesterol.31,80
The pleiotropic effects of statins in CVD are aid to a variety of methodology at both the sub-nuclear and cell levels. Some of these supportive effects of statins join change in endothelial cell work, limitation of platelet start/accumulation, block of VSMC migration and extension, lessened vascular aggravation, and extended soundness of atherosclerotic plaques. With these pleiotropic impacts in mind, the signs for statin use may continue growing, as the frameworks for these effects are totally outlined.
None.
Authors declare that there is no conflict of interest(s).

©2018 Sharma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.