eISSN: 2377-4304


Research Article Volume 13 Issue 6
1PRONATAL Clinic (Hospital Bité Médica) Mexico
2Women’s Health Clinic Mexico
3Clinical fertility horizons
4FertiFetal Clinic, Women’s/Reproductive/Prenatal Health
Correspondence: Lujan Irastorza Jesus Stuart, PRONATAL Clinic (Hospital Bité Médica) Mexico
Received: October 31, 2022 | Published: November 28, 2022
Citation: DOI: 10.15406/ogij.2022.13.00677
Introduction: Thrombophilias during pregnancy are associated with maternal-fetal morbidity and mortality. In addition to this, the physiological changes that arise during pregnancy also generate a state of hypercoagulability, which can lead to complications during pregnancy such as Fetal Growth Restriction (FGR), Preeclampsia and Gestational Loss (GL). The objective was to evaluate the efficacy of Metafolin (MF) against Folic Acid (FA), in pregnant women with MTHFR-C677T mutation.
Material and method: Retrospective, observational and cross-sectional study, which included 73 pregnant women. Groups: 1) GAF-T: Treatment with Folic Acid (FA, 400 mcg/24h) and 2) GMf-T: Treatment with Metafolin (Mf, 0.71 g/24h). In all cases, the women had the MTHFR C677T mutation and were treated with Heparin (5000 IU/12h) and Aspirin Protect (100 mg/24h), from the first trimester of pregnancy. Anthropometric data collection (in mothers and their newborns), presence of complications during pregnancy, MTHFR-C677T single nucleotide polymorphism (SNP) study and placental pathology were evaluated.
Results: The prevalence of Fetal Growth Restriction (FGR) (15.3 vs 11.1%), placental abruption (PA) (7.6 vs 5.5%), hypertension (7.6 vs 0%) and preeclampsia (7.6 vs 5.5%) in GAF-T and GMf-T was low. GMf-T presented fewer small villi (61.5 vs 22.2%), ischemic changes (76.9 vs 22.2%), erythrocyte extravasation (61.5 vs 22.2%) and hematomas (46.1 vs 11.1%).
Conclusion: The application of Mf from the beginning of pregnancy decreases the probability of developing placental pathologies. In addition, the joint application of Heparin and Aspirin Protect reduces the risk of developing complications during pregnancy such as Fetal Growth Restriction (FGR), Placental Abruption (PA), Hypertension and Preeclampsia.
Keywords: pregnant patients, uterus, prematurity, malformations
Pregnancy is a stage in which a proper fusion of the sperm with the oocyte, a good embryonic development and the achievement of the implantation of the blastocyst in the uterus. In addition, it is also the time when fetal development begins, which can be influenced by several factors both positive and negative.1 At this time, the nutritional status of the mother is of great importance because the deficiency of nutrients such as vitamin B, folates and vitamin B12 have been linked to alterations in pregnancy, among which are: low birth weight, prematurity, malformations, among others.1
Thus, folate deficiency is associated with: neural tube defects (NTDs), pregnancy-related complications, congenital heart defects, orofacial clefts, cardiovascular diseases, psychiatric diseases and cancer, which is why it is suggested that during pregnancy mothers have folic acid (FA) supplementation.1,2
On the other hand, studies show the existence of polymorphisms (thrombophilias) that affect the folate cycle, such as the mutation of methylenetetrahydrofolate reductase C677T (MTHFR-C677T), which increases homocysteine concentrations in blood, which leading to increased thromboxane production favoring platelet aggregation and the development of venous thrombosis events.3–8 Even, thrombophilias during pregnancy are related to maternal-fetal morbidity and mortality, due to the physiological changes that arise during this stage, which causes a state of hypercoagulability that makes women more prone to hypercoagulation and are also susceptible to pathological processes such as deep vein thrombosis.9–11
This can generate complications during pregnancy, among which are: alterations in early and late embryogenesis, increased risk of preeclampsia, Gestational Loss (GL), Fetal Growth Restriction (FGR), placental abruption (PA) and neonatal death, among others.12–15
In parallel, metafolin (Mf) (stable calcium salt of L-5-methyltetrahydrofolate acid, L-5-MTHF) is the most active form of reduced folate circulating in plasma and directly entering the metabolic process of folate. After administration, it shows optimal absorption, comparable or superior bioavailability, as well as physiological activity compared to FA.2,16
Mf supplementation is effective in pregnant and lactating women or women who wish to conceive, because it decreases plasma homocysteine and also increases the folate levels in plasma and erythrocytes. In addition, the administration of Mf skips the multistage reduction process before entering the folate cell cycle, this limits a possible deficiency of activity of the enzymes involved in the folate reduction process in the intestinal epithelium (DHFR and MTHFR), so it could replace folic acid in patients with MTHFR enzyme polymorphisms, because these mutations would prevent folic acid (FA) from being assimilated.16
The aim of this study is to compare the efficacy of Mf versus FA. Evaluating:
Retrospective, observational, cross-sectional study, which included 73 women who in the years 2020 and 2021 followed up their pregnancy at the PRONATAL clinic, which is located in the Hospital Bité Médica, Mexico City.
To carry out this study, 2 groups were formed: 1) GAF-T: Patients with FA treatment (400mcg/24h) throughout pregnancy and with thrombophilia study and 2) GMf-T: Patients with Mf treatment (0.71g/24h) throughout pregnancy. In both cases the patients were positive for MTHFR C677T thrombophilia (homozygous and heterozygous results were combined in a single parameter) and from the first trimester they were treated with Heparin (5000IU/12h) and Aspirin Protect (100mg/24h).
The collection of anthropometric data of mothers and newborns, such as age, weight (first trimester of pregnancy), height and BMI, as well as the weeks of gestation (WG) APGAR minute 1 and 5 (APGARm1 and 5), were taken from the medical record. This information was collected by the nursing staff during the first consultation of each patient.
The analysis of thrombophilias were performed by taking a peripheral blood sample, which was sent to the laboratory of the Institute of Human Reproduction Sciences, where it was analyzed if they presented single nucleotide polymorphisms (SNP) of MTHFR-C677T using the polymerase chain reaction technique.
The placentas were processed at the Department of Medical Bité Pathology. Common sections were fixed in formalin, processed into paraffin, and stained with hematoxylin/eosin.
All women were informed about the use and management of the data collected, allowing their inclusion in this study. In addition, their anonymity is maintained, as they do not refer to the origin of the information and only numerical and statistical data (as the case may be) are disclosed.
Inclusion criteria: Women who had their delivery at the Bité Médica Hospital and who throughout pregnancy were treated with Aspirin Protect and Heparin, in addition to FA or Mf, with study to identify MTHFR-C677 SNPs, complete records [age, weight, height (mother and child) and body mass index (BMI) of the mother, in addition, WG, APGAR (minute 1 and 5)], complications that occurred during delivery (FGR, PA, Hypertension, Preeclampsia, GL and Death) and results of placental pathology.
Exclusion criteria: Women who did not accept inclusion in the study.
Statistical analysis
Age, weight, height and BMI (mother and baby) are reported with mean ± standard deviation (SD) and the presence of significant difference between groups was evaluated using student's T test (p<0.05) and Shapiro-Wilk normality test, in addition, SDG and APGAR m1 and m5, are reported with mean ± standard deviation (SD). On the other hand, the thrombophilia evaluated (MTHFR-C677T), perinatal complications (IUGR, PA, hypertension, preeclampsia, GL and death), as well as the prevalence of placental pathology, were expressed in percentages and the difference between groups was evaluated by performing a chi2 test (p<0.05). In both cases, the statistical package SPSS, version 25, was used.
This study included 73 women who had their delivery at the Bité Médica Hospital in 2020, who, when comparing GAF-T with GMf-T, showed no statistically significant difference in age, weight, height and BMI (Table 1).
|
n |
Age (years) |
Weight (kg) |
Height (m) |
BMI (kg/m2) |
GAF-T |
40 |
34.5±4.7 |
60.2±8.5 |
1.63±0.07 |
22.6±3.3 |
GMf-T |
33 |
35.8±4.8 |
61.5±10 |
1.60±0.05 |
23.9±3.4 |
p |
|
0.7 |
0.6 |
0.3 |
0.8 |
Table 1 Anthropometric data of pregnant women in the first trimester
Student’s t-test, p<0.05.
When the prevalence of thrombophilias were assessed, a higher prevalence of MTHFR-C677T (54.5 vs 32.5%) was found in GMf-T compared to GAF-T (Graph 1).
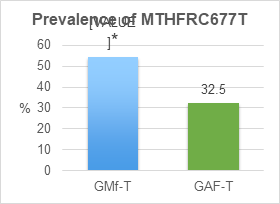
Figure 1 Shows the prevalence of MTHFR-C677T SNPs, in GAF-T and GMf-T. Statistical difference, when comparing GAF-T vs GMf-T (Chi2 test and p<0.5).
Regarding the perinatal complications that occurred during pregnancy, they showed no significant difference in the prevalence of FGR (15.3 vs 11.1%), PA (7.6 vs 5.5%), hypertension (7.6 vs 0%) and preeclampsia (7.6 vs 5.5%) when comparing GAF-T with GMf-T (Graph 2).
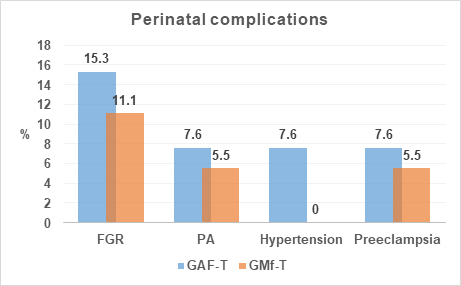
Figure 2 Shows prevalence of complications that occurred during pregnancy. FGR: Fetal Growth Restriction, PA: Placental Abruption, Hypertension and Preeclampsia. Chi2 test and p<0.5, there were no statistical changes.
Similarly, when the anthropometric data of newborns were evaluated, no significant difference was found between Weight (2878.05±478.4 vs 2847.3±445.8) and Height (47.8±2.1 vs 47.7±2.2, p≥0.05), in addition, both groups maintained similar WG (38.4±1.1 vs 37.8±1.3), APGAR m1 (8.9±0.2 vs 8.8±0.4) and APGAR m5 (9.5±0.5 vs 9.6±0.4) values (Table 2).
|
n |
WG |
Weight |
Height |
APGAR 1m |
APGAR 5m |
GAF-T |
40 |
38.4±1.1 |
2878.05±478.4 |
47.8±2.1 |
8.9±0.2 |
9.5±0.5 |
GMf-T |
33 |
37.8±1.3 |
2847.3±445.8 |
47.7±2.2 |
8.8±0.4 |
9.6±0.4 |
p |
|
- |
≥0.05 |
≥0.05 |
- |
- |
Table 2 Anthropometric data of newborns
Student’s t-test, p<0.05.
After the collection and pathological analysis of placentas, a significant increase was found in small chorionic villi GAF-T (61.5 vs 22.2%), IC (76.9 vs 22.2%), EE (61.5 vs 22.2%) and hematomas (46.1 vs 11.1%), compared to GAF-T. In addition, numerical increase in GAF-T of atrophic chorionic villi (38.4 vs 22.2%), DS (38.4 vs 22.2%), CDC (76.9 vs 44.4%), fibrin (53.8 vs 27.7%), hemorrhage (30.7 vs 16.6%), edema (15.3 vs 11.1%), OT (15.3 vs 5.5%) and infarct (38.4 vs 22.2%), compared to GMf-T (Graph 3).
Finally, no statistical increase was observed, but there was a numerical increase in the prevalence of Umbilical Cord (UC), edema (30.7 vs 5.5%), hematoma (7.6 vs 0%), hemorrhage (15.3 vs 5.5%) and EE (23.07 vs 5.5%), in GAF-T when compared with GMf-T (Graph 3).
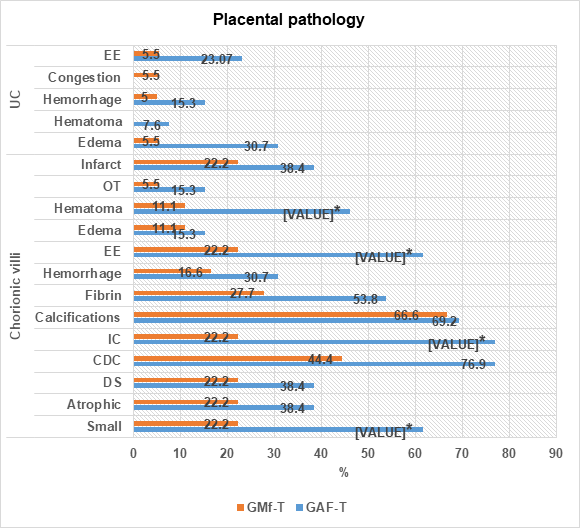
Figure 3 Shows prevalence of placental pathology (chorionic villi and umbilical cord): Patients treated with GAF-T vs GMf-T. FA: Folic Acid, Mf: Metafolin, OT: Organized Thrombi, EE: Erythrocyte Extravasation, IC: Ischemic Changes, CDC: Congestion of Dilated Capillaries and DS: Dense Stroma. *Statistical difference when comparing GMf-T with GAF-T, Chi2 test and p<0.05.
In Mexico there are few studies that report prevalence of thrombophilias in the Mexican population. Two of these studies are those conducted by Luján J. et al.,17 in 2020 and 2021, who in the population of patients of the PRONATAL clinic showed a high prevalence of MTHFR-C677T polymorphism (48.9%).17,18 On the other hand, in this study we found that GAF-T (32.5%) and GMf-T (54.5%) presented a high prevalence of MTHFR-C677T, being higher in GMf-T. This has been associated with complications during pregnancy, such as alterations in early and late embryogenesis, increased risk of Preeclampsia, hypertension and miscarriage, FGR, PA, GL, etc.19–26
In this case, FGR was the alteration that occurred the most, both in GAF-T (15.3%) and GMf-T and (11.1%) (Graph 2), presenting figures lower than those reported by authors such as Coriu et al.,27 who report that 34.4% of patients with MTHFR C677T developed FGR.27 Similarly, Dugalic et al., 2018, reported that 27.3% of patients with MTHFR C677T mutation developed FGR. In the case of PA [7.6% (1/13) vs 5.5% (1/18)], hypertension [7.6% (1/13) vs 0% (0/18)] and preeclampsia [7.6% (1/13 vs 5.5 (1/18)], we observed very low and similar prevalence when comparing GAF-T with GMf-T (Graph 2), different from that found by Azimi et al.,29 who observed that in a group of 125 patients with MTHFR-C677T SNPs, 62.4% developed preeclampsia, as did Mislanova et al.,30 show high prevalence of preeclampsia (45.7%) in a group of 35 patients with MTHFR C677T mutation. For his part, Karakantza et al.,31 in a study that included 15 women with PA, report high incidence of the MTHFR C677T mutation (40%), similar to Gebhardt et al.,32 which included 18 women with PA, found MTHFR C677T prevalence of 28%. In parallel, Song et al.,33 report in a group of 306 patients with hypertension MTHFR-C677T prevalence of 77.7%. In turn, Ya et al.,34 in systematic review, found prevalence of 2.6 to 47.06% of the MTHFR-C677T polymorphism in 8064 women with hypertension. In addition to this, no significant difference was found in the anthropometric data of newborns between GAF-T and GMf-T, reporting gestation week or GW (38.4±1.1 vs 37.8±1.3), Weight (2878.05±478.4 vs 2847.3±445.8), Height (47.8±2.1 vs 47.7±2.2), APGAR 1m (8.9±0.2 vs 8.8±0.4) and APGAR 5m (9.5±0.5 vs 9.6±0.4).
In GAF-T and GMf-T, the low incidence of FGR, PA, hypertension and preeclampsia, in addition to the normality of the anthropometric data of newborns, may be the result of the application of heparin combined with aspirin protect as a preventive treatment. Both agents have anticoagulant properties, but work differently: Heparin increases the effect of the natural antithrombin; while aspirin inhibits platelet aggregation.35,36 Similarly, the application of folic acid could have a protective effect, since the presence of the MTHFR C677T mutation only decreases the activity of the MTHFR enzyme by 35 to 70% in patients who present it.
On the other hand, in the literature the increase in placental alterations has been related to the development of alterations such as FGR, PA, hypertension and preeclampsia, found in placentas of patients with these alterations (such as) calcifications (32.7%), infarct (5 to 72.7%), thrombi (32 to 45.3%), hemorrhage (85%), edema (91.8%), chronic villitis (21%), chronic chorioamnionitis (5.3 to 36%), chronic deciduitis (98%), hairy fibrinoid necrosis (72.7%), membrane necrosis (20%), elevated nucleated red blood cells (89%), increased syncytial nodes (93%), distal villus hypoplasia (35%), placental hypermaturity (40%), and decidual vasculopathy (30.6%).37–40
In this study, despite having observed a low prevalence of FGR, PA, hypertension and preeclampsia, our results show the presence of placental alterations, such as chorionic villi small (61.5 vs 24.2%) and atrophic (38.4 vs 22.2%), with IC (76.9 vs 22.2%), EE (61.5 vs 22.2%), hematomas (46.1 vs 11.1%), DS (38.4 vs 22.2%), CDC (76.9 vs 44.4%), fibrin (53.8 vs 27.7%), hemorrhage (30.7 vs 16.6%), edema (15.3 vs 11.1%), OT (15.3 vs 5.5%) and infarct (38.4 vs 22.2%). In addition to an increase in UC edema (30.7 vs 5.5%), hematoma (7.6 vs 0%), hemorrhage (15.3 vs 5.5%) and EE (23.07 vs 5.5%), in GAF-T and GMf-T, respectively (Graph 3). Placental alterations were greater in GAF-T, compared to GMf-T, may be because FA in GAF-T requires activation by reduction before it can serve as a coenzyme for one-carbon transfer reactions. This activation results in the reduction of 5,10-methylenetetrahydrofolate to L-5-methylenetetrahydrofolate [Metafolin (Mf)] and has been observed to affect up to 70% of patients with MTHFR C677T polymorphism.41,42 Therefore, the direct implementation in the diet of Mf (GMf-T) as a treatment, restores the folate cycle of this group of patients allowing the decrease of homocysteine levels, avoiding the activation of thromboxanes, formation of clots and thrombi, protecting against the development of placental alterations where FA is not, in theory, effective.43
Preventive treatment with FA or Mf throughout pregnancy, combined with the application of heparin and aspirin protect from the first trimester, decreases the risk of developing complications such as FGR, PA, hypertension and preeclampsia in pregnant women with the presence of the MTHFR C677T mutation.
Mf compared to FA, as a preventive treatment throughout pregnancy more effectively reduces the risk of developing placental pathologies such as:
Edema, hematomas, hemorrhage and erythrocytes extravasation.
Finally, prospective studies with a larger analysis population are necessary, not only to evaluate the presence of the MTHFR C677T mutation, but also to include a more complete panel of inherited thrombophilias.
Limitations
Simultaneously to treatment with AF and Mf, patients received treatment with aspirin protect and heparin. This masked the possible benefits for reducing complications during pregnancy by applying AF or Mf.
None.
None.
There are no conflicts of interest.

©2022 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.