eISSN: 2377-4304


Research Article Volume 11 Issue 4
1Clínica de Ginecología y obstétrica (PRONATAL), Mexico
2Clínica de Salud Femenina, Mexico
Correspondence: Vargas Hernández Víctor Manuel, Clínica de Salud Femenina, Mexico, Tel (52) 5552179782, Fax (52)5555746647
Received: May 21, 2020 | Published: July 16, 2020
Citation: Estuardo LIJ, Jesús APF, Daniela AR, et al. Incidence of hereditary thrombofilies in a population of Mexican women. Obstet Gynecol Int J. 2020;11(4):208-213. DOI: 10.15406/ogij.2020.11.00511
Objective: To report the incidence of thrombophilias and importance in the development of thrombotic events in a population of Mexican women.
Methods: Is a retrospective, observational and cross-sectional study of 184 women of reproductive age, where the age, weight, height and study of hereditary thrombophilias of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G were studied. Four groups were formed: 1) FVL-G1691A, 2) PT-G20210A, 3) MTHFR-C677T and 4) PAI-1 4G/5G, each group was separated by homozygous and heterozygous mutation.
Results: MTHFR-C677T and PAI-1 4G/5G present higher incidence (48.9 and 64%), when comparing with FVL-G1691A and PT-G20210A (3.8 and 0.5%) (p<0.05), higher incidence of PAI-1 4G/5G was observed, when compared to MTHFR-C677T (64.6 vs. 48.9%, p<0.05), difference that was not observed when comparing FVL-G1691A with PT-G20210A (3.8 vs. 0.5%, p>0.05). When patients presented only one thrombophilia, the highest incidence is of MTHFR-C677T and PAI-1 4G>5G (16.5 and 35.2%). Patients with multiple thrombophilias had an incidence of MTHFR-C677T with PAI-1 4G/5G of 30.2%.
Conclusion: Our results in the population of Mexican women, we report a high incidence of the MTHFR-C677T and PAI-1 4G / 5G mutation, which makes them susceptible to the development of thrombotic events.
Keywords: thrombophilia, miscarriage, thrombosis, coagulation, deep vein thrombosis, mutation
Thrombophilia is defined as an abnormal tendency to form venous thrombosis that can result in deep vein thrombosis.1
The clinical pattern of deep vein thrombosis shows wide heterogeneity, although they have the same thrombophilia defect. Common risk factors associated with deep vein thrombosis are obesity, smoking, blood group (ABO), and polymorphisms. The distinct thrombophilias causes different severities in the tendency to thrombosis and not all can be treated in the same way. Furthermore, more than one predisposing condition exists in the same individual, example of this are other defects such as protein S deficiency, caused by more than one genetic mutation. These different mutations differ in their phenotypic presentations, and other factors.2
Thrombophilias in pregnancy are related to maternal-fetal morbidity and mortality, due to the physiological changes of pregnancy, which is a state of hypercoagulability, making women more prone to hypercoagulation, and susceptible to pathological processes such as deep vein thrombosis. The incidence of maternal mortality is reported in emerging countries of 1 in 1000, with the greatest increase during the puerperium;3–5 where, mainly, they can develop venous thrombosis or deep vein thrombosis, particularly, with a higher incidence in the lower limbs than in the arms and abdomen,6 it is associated with circulatory and cardiac problems, alteration of reproductive functions and failure in embryo implantation. Thrombophilias can be acquired; such as antiphospholipid syndrome, hyperhomocysteinemia (non-genetic), hypothyroidism, increased activity in clotting factors such as IX, XI or VIII, deficiency of plasminogen and acquired resistance to activated protein C and inherited thrombophilias, such as polymorphism of a single nucleotide of factor V Leiden (FVL-G1691A) and prothrombin (PT-G20210A), overexpression leads to activation of thrombin, which is a protein involved in the hemostasis system for clot formation (hypercoagulability) (Figure 1).7–9
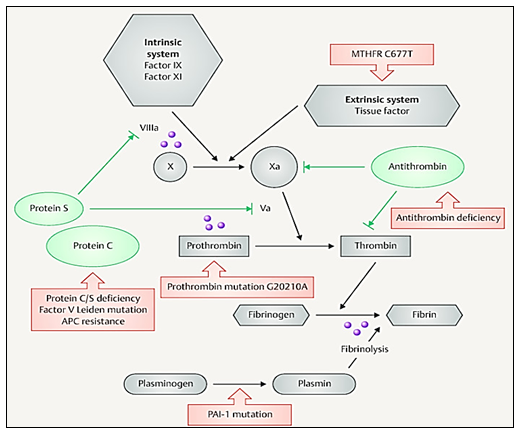
Figure 1 Shows the level in which FVL (Va), Prothrombin, MTHFR and PAI-1 participate. Image obtained from Stefanski A, et al.,9
These two thrombophilias are more common and associated with an increased risk of developing deep vein thrombosis (symptomatic or asymptomatic) and pulmonary thromboembolism.10
Exist other thrombophilias that have an impact on the formation of venous thrombosis, not only because of their participation as an activator in the coagulation cascade, but also because some of these factors inhibit coagulation and, being inactive or blocked, allow the formation of clots,4,11–14 is the case of the methylenetetrahydrofolate reductase mutation (MTHFR-C677T) and the plasminogen activator-1 inhibitor (PAI-1 4G/5G).
MTHFR-C677T develops an increase in homocysteine concentrations, which increases the production of thromboxanes that favor platelet aggregation and the development of venous thrombosis (Figure 2).15–18

Figure 2 Shows the participation of MTHFR in the folate cycle. MTHF, 5 methyltetrahydrofolate; MTHFR SNP, methylene tetrahydrofolate reductase singlenucleotide polymorphism; SAH, S adenosyl homocysteine; SAM, S adenosyl methionine; THF, tetrahydrofolate. Image taken from Goyco L, et al.,18 SAH (S adenosyl homocysteine) and SAM (S adenosyl methionine).
In turn, PAI-1, is a factor that participates in fibrinolysis, its PAI-1 4G/5G mutation increases levels of PAI-1 in plasma allowing the formation of clots and the possible generation of venous thrombosis (Figure 1).9,17,19,20– 22 In addition to this, there are studies that report that the presence of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 5G> 4G, can generate complications during pregnancy, such as alterations in early and late embryogenesis, increased risk of preeclampsia, early loss of pregnancy, intrauterine growth restriction, premature placental abruption, fetal, neonatal death, and recurrent gestational loss.8,23– 27
The objective of this work is to report the incidence of these four thrombophilias and their importance with respect to the development of thrombotic events in a population of Mexican women.
It is a retrospective, observational and cross-sectional study, where 184 Mexican women of reproductive age were included, which were attended in the gynecology and obstetrics clinic (Pronatal), during the period 2015-2018. Inclusion criteria: Women who underwent a study of hereditary thrombophilias (FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI -1 4G/5G), including pregnant or nonpregnant women with a history of gestational loss, preeclampsia, intrauterine growth restriction, stillbirth, placental abruption, neonatal death, family background, and uncomplicated term pregnancies. Exclusion criteria: patients with non-inherited thrombophilias.
Four groups were formed with the obtained data: 1) FVL-G1691A, 2) PT-G20210A, 3) MTHFR-C677T and 4) PAI-1 4G/5G. Additionally, each group was separated by homozygous and heterozygous mutation: FVL-G1691A (homozygous=AA and Heterozygous=GA), PT-G20210A (Homozygous =AA and Heterozygous=GA), MTHFR-C677T (Homozygous=TT and Heterozygous=CT) and PAI-1 4G/5G (homozygous=4G/4G and heterozygous=4G/5G). Age, weight and height were studied in the first consultation.
The analysis of the thrombophilias was carried out by taking a peripheral blood sample, and it was sent to the laboratory of the Institute of Human Reproduction Sciences, where it was analyzed if they presented polymorphism of a single nucleotide, polymorphism of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G, using the polymerase chain reaction technique.
All women were offered study information and asked for their letter of informed consent; on the use and management of data privacy (age, weight, height, and thrombophilic results) to be included in the study. The inclusion criteria were women of reproductive age, with a study of thrombophilias (FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G); complete files (age, weight, height and thrombophilias study), study of positive and negative thrombophilias. Exclusion criteria were women without study of thrombophilias or who did not accept their inclusion in the study. Age, weight and height are reported with mean ± standard deviation. On the other hand, the difference in incidence between the different thrombophilias and the homozygous vs. heterozygous version was performed with a chi2 using the SPSS version 25 statistical package.
The analysis of our results showed that the polymorphism of MTHFR-C677T and PAI-1 4G/5G, presented a higher incidence of 48.9% and 65% respectively, where the homozygous form represented 16.8% (MTHFR-C677T) and 21.7% (PAI-1 4G/5G) and the heterozygous form 32% (MTHFR-C677T) and 42.9% (PAI-1 4G/5G). The high incidence of MTHFR-C677T and PAI-1 4G/5G represents a risk, because Mexican women may have a greater predisposition to develop thrombosis; Table 1 gives information on the age, weight, height, and body mass index (BMI) of 184 women who were included in the study.
Population characteristics |
|
Age (years) |
33.6±5.1 |
Weight (Kg) |
60.7±10.2 |
Height (cm) |
167.8±6.3 |
BMI (Kg/m2) |
21.5 |
Table 1 Shows population size. Age, weight, height and BMI (mean±SD)
In Figure 3, it is observed that MTHFR-C677T and PAI-1 4G/5G have a higher incidence (48.9 and 64%), when compared with FVL-G1691A and PT-G20210A (3.8 and 0.5%) (p<0.05). In addition, a higher incidence of PAI-1 4G/5G was observed, when compared with MTHFR-C677T (64.6 vs. 48.9%, p<0.05), difference that was not observed when comparing FVL-G1691A with PT-G20210A (3.8 vs. 0.5%, p>0.05).
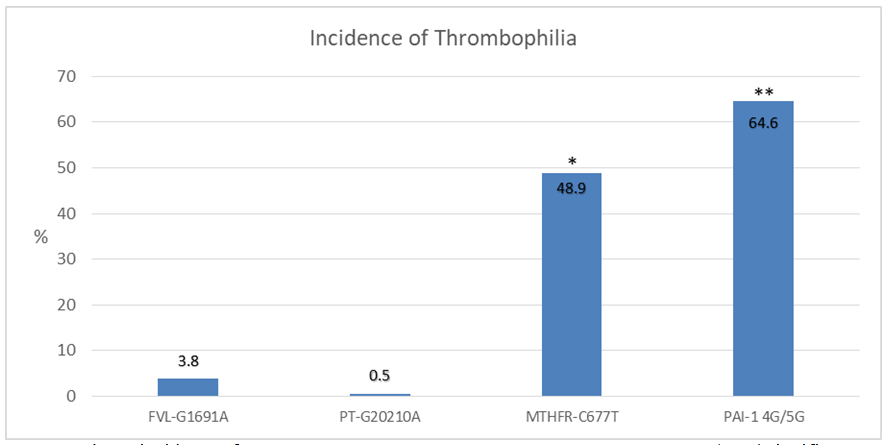
Figure 3 Shows incidence of FVL-G1691A, PT-G20210A, MTHFR-C677T y PAI-1 4G/5G. *Significant difference (p˂0.05) when comparing with MTHFR-C677T vs FVL-G1691A y PT-G20210A. ** Significant difference (p˂0.05) when comparing with PAI-1 G4/5G vs MTHFR-C677T, FVL-G1691A y PT-G20210A.
It is shown in Figure 4, that MTHFR-C677T (16.8 vs. 32, p<0.05) and PAI-1 4G/5G (21.7 vs. 42.9, p<0.05), have a higher incidence in their Heterozygous form when compared with the Homozygous. In the case of the FVL-G1691A (1.6 vs 2.1, p>0.05) and PT-G20210A (0 vs 0.5, p>0.05), they did not present a significant difference between its homozygous and heterozygous form; Figure 3, Shows incidence of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G* Significant difference (p˂0.05) when comparing MTHFR-C677T versus FVL-G1691A and PT-G20210A **. Significant difference (p˂0.05) when comparing PAI-1 G4>5G versus MTHFR-C677T, FVL-G1691A and PT-G20210A. In Figure 5, it describes when the patients presented only one thrombophilia, the highest incidence is of MTHFR-C677T and PAI-1 4G/5G (16.5 and 35.2%), different from FVL-G1691A and PT-G20210A, which were not presented individually in no patient. An incidence of MTHFR-C677T with PAI-1 4G/5G of 30.2% was seen in patients who had multiple thrombophilias. On the other hand, FVL-G1691A and PT-G20210A were only evident in combination with MTHFR-C677T and / or PAI-1 4G/5G, without exceeding 1.5%. Finally, the percentage of patients who did not present any of these four thrombophilias was 13.6%. Figure 4 shows incidence of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G, in its heterozygous and homozygous form* Significant difference (p˂0.05) when comparing homozygous vs. heterozygous MTHFR-C677T **. Significant difference (p˂0.05) when comparing homozygous versus heterozygous PAI-1 G4/5G. Figure 5 shows the incidence of combinations of thrombophilias presented by the population.
Thrombophilias and their development of thrombotic events in a Mexican female population; There are many studies carried out on the incidence of thrombophilias, however, in Mexico there is still no representative sample that indicates the trend of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G, which has great importance for our environment this study.
For its part, the analysis of our results showed that the polymorphism of MTHFR-C677T and PAI-1 4G/5G, present a high incidence of 48.9% and 65% respectively, where the homozygous form represented 16.8% (MTHFR-C677T) and 21.7% (PAI-1 4G/5G) and the heterozygous form 32% (MTHFR-C677T) and 42.9% (PAI-1 4G/5G); with similar results in different regions of Europe and Asia.26,30–35
The high incidence of MTHFR-C677T and PAI-1 4G/5G represents a risk, because Mexican women may have a greater predisposition to develop venous thrombosis and deep vein thrombosis, due to the alteration of the coagulation mechanisms induced by these thrombophilias. Reports in studies where high homocysteine concentrations of up to 24µmol/L and lack of fibrinolysis inhibition have been associated with the development of venous thrombosis and deep vein thrombosis.36–40 Additionally, some studies of Patients with venous thrombosis or deep vein thrombosis have reported an incidence of MTHFR-C677T of up to 35%, showing that both the control group and the group with venous thrombosis or deep vein thrombosis have similar incidences.41–43
This is due to the fact that thrombosis or deep vein thrombosis have different risk factors44 6, other report on PAI-1 4G/5G, associate the development of venous thrombosis or thrombosis deep venous mainly to the homozygous form (25.8 versus 14.7%), which was the only one that presented a significant difference when compared with the control group.45
In turn, FVL-G1691A and PT-G20210A, presented an incidence of 3.8% and 0.5% respectively, where the homozygous form represented 1.6% (FVL-G1691A) and 0% (PT-G20210A) and the heterozygous form 2.1% (FVL -G1691A) and 0.5% (PT-G20210A). These values are lower than studies carried out in other populations in Europe, Asia and North America (Caucasian populations), which have an incidence of FVL-G1691A in its heterozygous form of 22.8% and its homozygous form of up to 3%, and of PT- G20210A up to 10% in its heterozygous form and 0.6% in its homozygous form.46–57 Also, the presence of FVL-G1691A is associated with the development of venous thrombosis and deep vein thrombosis, as reported. As shown,58 in patients with deep vein thrombosis they have a higher rate of this mutation when compared with the control group (18 vs. 3.8%); others identify. For their part,59,60 an incidence of 54% of FVL-G1691A in patients with deep vein thrombosis; a review,61 show an increase in the rate of FVL-G1691A in patients with venous thrombosis where values of 52% are shown compared to the control group that presented values of only 14.6%, clearly expanding the knowledge of the role of FVL-G1691A in the development of venous thrombosis or deep vein thrombosis, another report. Attia F, et al., in 2009, observed the relationship between PT-G20210A and the development of deep vein thrombosis, in patients with deep vein thrombosis; a meta-analysis reports that patients with venous thrombosis have a higher incidence of PT-G20210A with values of up to 21.9% compared to the control group that presented values62 of up to 6.1%. Regarding the presence of multiple thrombophilias per patient,63 reported an incidence of 13.3% of multiple thrombophilias per patient, without specifying the type of thrombophilias, which was not associated with the development of venous thrombosis. profound, it is different from our results that we had a low incidence of patients with combination of MTHFR-C677T or PAI-1 4G/5G+FVL-G1691A or PT-G20210A ranging from 0.7 to 1.4%, and a high incidence of the combination of MTHFR-C677T+PAI-1 4G/5G which was 30.2%. Of these results, 0.7 of the patients presented a combination of MTHFR-C677T and PT-G20210A, a combination that was associated,64 with the, in a 56-year-old patient, in which their parents, siblings and children presented this combination of thrombophilias, of which 4 out of 6 had pathologies related to the coagulation system.
Finally, as can be seen, there are a large number of studies that relate the presence of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G with the development of venous thrombi or deep vein thrombosis, it is important to highlight that the presence of any of these thrombophilias is not always indicative of the development of an alteration in hemostasis in favor of the development of venous thrombi or deep vein thrombosis, in many cases it is the mixture of factors that are part of the picture presented by patients who develop thrombotic events , such as oral contraceptives, the physiological changes of pregnancy and the puerperium with a state of hypercoagulation, hormone therapy for menopause, aging, sedentary lifestyle, obesity, surgery, serious infections, cancer, etc.44
Study limitations
The number of patients is low to represent the Mexican population, due they are only data obtained from a single health center. Even so, we believe that the study design shows a trend of the thrombophilias evaluated, which can be taken as a basis for future studies.
The population of Mexican women studied has a high rate of MTHFR-C677T and PAI-1 4G/5G, which makes them more susceptible to the development of thrombotic events. Furthermore, FVL-G1691A and PT-G20210A are of low incidence; but, it is combined with MTHFR-C677A Y/o PAI-1 4G/5G, increasing the effects of these thrombophilias; further studies on thrombophilias and the relationship with thrombotic events in Mexican women are required.
None.
None.
The authors declare there are no conflicts of interest.

©2020 Estuardo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.