eISSN: 2377-4304


Case Report Volume 9 Issue 4
1Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, USA
2St. George's University, School of Medicine, Grenada
3Key Laboratory for Major Obstetric Diseases of Guangdong Province, Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Guangzhou Medical University, China
4Maternal and Fetal Medicine, Department of Obstetrics and Gynecology, Lincoln Medical and Mental Health Center, USA
5Division of Minimally Invasive Gynecologic Surgery, Department of Obstetrics and Gynecology, Baylor College of Medicine, USA
Correspondence: Juan Liu MD PhD, Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, China, Tel 86-20-81292697, Fax 86-20-81292697, Tel (832) 826-7464, Fax (832) 825-9349
Received: July 09, 2018 | Published: August 16, 2018
Citation: Rezai S, Hughes AC, Zeng C, et al. Laparoendoscopic single-site oophorectomy for a right ovarian borderline serous tumor of stage IC2, a literature review. Obstet Gynecol Int J. 2018;9(4):301-304. DOI: 10.15406/ogij.2018.09.00351
Background: Ovarian cancer represents approximately 30% of all female reproductive organ cancers and is the leading cause of death for gynecological cancer in the United States. Borderline ovarian tumors comprise approximately 15-20% of all epithelial ovarian tumors and within that group, serous borderline ovarian tumors comprise 53%. Serous borderline tumors (SBT) display atypical epithelial proliferation of serous type cells, outnumbering benign epithelial cells but have a low malignancy potential (borderline). By definition SBT has no gross stromal invasion but it can be subcategorized to those with or without microinvasion. Carcinomas make up 90% of ovarian tumors and differentiating between the distinct types of tumors via unique features is important for treatment.
Case: The patient was a 29-year-old G0P0, who presented to our clinic for evaluation of intermenstrual bleeding. Upon physical exam, a palpable, 5cm x 4cm non-tender, mobile mass in the right adnexa was noted. Pelvic ultrasound revealed a 4.9cm x 3.7cm hypoechoic mass in the right adnexa. The mass was noted to be heterogeneous with smooth borders and a preliminary diagnosis of ovarian cystadenoma was made. The patient in this case presented with a stage 1C2 ovarian cancer and was treated with single incision laparoscopic surgery (SILS) right salpingo-oophorectomy.
Conclusion: Ovarian cancer is the leading cause of gynecological cancers in women in the US and early diagnosis/staging is important in predicting progression-free survival. Instead of laparotomy, we utilized single-incision laparoscopic surgery to drain the patient’s pelvic effusion and excise the right ovary and fallopian tube, which revealed an ovarian borderline serous tumor. This technique was conservative and fertility-sparing because it left the uterus, left ovary and left fallopian tube.
Keywords: borderline serous tumor, early stage ovarian cancer, epithelial tumor, oophorectomy, ovarian cancer, ovarian cyst, ovarian epithelial tumor, ovarian tumor, single-incision laparoscopic surgery (SILS), stage I ovarian cance, serous tumor, stage IC2 ovarian cancer
Ovarian cancer represents approximately 30% of all female reproductive organ cancers and is the leading cause of death for gynecological cancer in the United States.1 Borderline ovarian tumors comprise approximately 15-20% of all epithelial ovarian tumors and within that group, serous borderline ovarian tumors comprise 53%.2,3 Serous borderline tumors (SBT) display atypical epithelial proliferation of serous type cells, outnumbering benign epithelial cells but have a low malignancy potential (borderline).4,5 By definition SBT has no gross stromal invasion but it can be subcategorized to those with or without microinvasion.4 Carcinomas make up 90% of ovarian tumors and differentiating between the distinct types of tumors via unique features is important for treatment.
SBT tumors are usually asymptomatic, but may present with abdominal enlargement or pelvic pain, and are related to a high rate of infertility in young women.1 The mean age of diagnosis of 1C ovarian tumor is 51.7 years.6 According to a long term follow up study, SBT tumors have a 5 year survival rate of 100% and a 10 year survival rate of 86%.1 The cure rate for serous borderline tumors is close to 100% especially when they are diagnosed at stage 1.2,7
Cancer staging allows for understanding of the patient’s condition, prognosis, and treatment options. Staging for ovarian cancer is guided by the International Federation of Gynecology and Obstetrics (FIGO) which was last updated in 2014.8 The patient in this case presented with a stage 1C2 ovarian cancer and was treated with single incision laparoscopic surgery (SILS) right salpingo-oophorectomy.
The patient was a 29-year-old Gravida 0, Para 0, who presented to our clinic for evaluation of intermenstrual bleeding. She endorsed regular menstrual cycles of 28 days with menses lasting 4-6 days and denied pelvic pain, dysmenorrhea and dyspareunia. Upon physical exam, a palpable, 5cm x 4cm non-tender, mobile mass in the right adnexa was noted. Pelvic ultrasound revealed a 4.9cm x 3.7cm hypoechoic mass in the right adnexa. The mass was noted to be heterogeneous with smooth borders and a preliminary diagnosis of ovarian cystadenoma was made.
Computed tomography (CT) of the pelvis (Figure 1) showed a cystic mass in the right adnexa suspicious for ovarian borderline epithelial tumor or cystadenocarcinoma. A small amount of free fluid in the cul-de-sac and endometrial thickening were also noted (Figure 1).
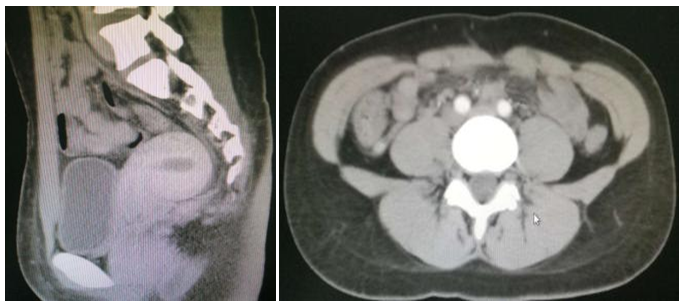
Figure 1 CT Scan of pelvis: The volume of the uterus is full, density of uterine wall is well-distributed , endometrium slightly thickened, enhanced scan show no lesions, solid-cystic hypodense shadow was seen in the right-sided adnexa, cystic lesion which is in the size of 4.3cm X 3.8cm X 5.3cm is predominant. In the cystic lesion, we can see line like separate shadow, papillary nodules can be seen in its top wall; papillary nodules are in the size of 1.0cm X 0.6cm X 0.6cm. The demarcation between the lesion and the bladder or adjacent intestine was clear. No lymphadenopathy noted.20
The patient underwent hysteroscopy, single incision laparoscopic surgery (SILS), right salpingo-oophorectomy (RSO), uterine surface nodule resection and endometrial curettage.
Hysteroscopy revealed a normal cervix and a normal sized uterus. The endometrium was pale red, thick, uniform with visible glandular openings and two pink tongue pattern lesions on the posterior wall. Endometrial curettage for the clinical diagnosis of endometrial hyperplasia was performed.
Single incision laparoscopy revealed a normal sized uterus and a small (20ml) clear yellow pelvic effusion which was drained and sent for cytology. Four endometriosis lesions, 2mm in size were observed on the posterior uterine wall and there were multiple white 3mm nodules on the anterior uterus close to the round ligament. The left ovary and bilateral fallopian tubes were normal. The right ovary was observed to have surface papillary lesions, which were excised and sent for frozen pathology and revealed to be an ovarian borderline serous tumor. The right ovary and fallopian tube were removed. Omentum and bilateral lymph nodes were removed for staging. Peritoneal washings were performed and sent for cytology which was negative.
Postoperative pathology of the right ovary revealed serous borderline tumors and corpus luteum cysts. (Figure 2) (Figure 3) The right fallopian tube was negative for malignancy. An endometrial specimen showed early secretory stage but no tumor involvement. The uterine surface nodules were determined to be leiomyoma. Omentum and bilateral lymph nodes were negative for metastasis. Therefore, with SBT limited to the surface of one ovary it was staged as 1C2, which is defined as a tumor that is on the outer surface of at least one of the ovaries or fallopian tubes or the capsule has ruptured before surgery.8
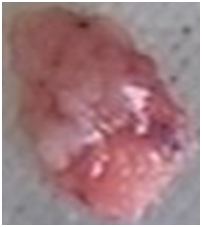
Figure 2 Pathology: Gross appearance of right ovarian tumor with papillary surface: Gray red tissue measuring 1cm X 0.7cm X 0.4cm
A drainage tube was placed in the subcutaneous fat which remained after surgery. There was plenty of lymph drainage in the immediate postoperative period. By postoperative day 7 the drainage had slowed to 50ml and was removed. The remainder of the patient’s recovery was uncomplicated and she was discharged on postoperative day 15.
On postoperative follow up visit, 4 months after the surgery, the patient reported regular menstrual cycles with complete resolution of intermenstrual bleeding, but complained of slight right adnexal tenderness just before start of her menses. She is being followed and monitored by serial tumor maker (AFP, CEA, CA125, CA153, CA199, CA50 and serum ferritin) and abdominal/pelvic ultrasound, which have been normal up to this date.
The patient presented with intermenstrual bleeding and although a mass was detected on physical exam the patient did not complain of any symptoms such as pelvic pain, heaviness or dysuria. This presentation is less common for SBT as it does not have any of the symptoms outlined in the 2007 ovarian cancer consensus statement.7 Of note, these recommendations have not shown to decrease mortality and were developed because ovarian disease was often found at late stages.7
Traditionally surgical staging for ovarian cancer has been done with laparotomy but advances in minimally invasive surgery have made endoscopic surgical staging more common. There has been concern of under-staging when laparotomy is not used but there is no conclusive evidence endoscopic surgery results in higher rates of recurrence or increased mortality.9,10 A study by Song et al. found multi port and single port laparoscopy to be a viable option for surgical staging of borderline ovarian tumors.9 In this case we used the SILS technique, which has shown to have improved outcomes for cosmetics and pain.11 Additionally, SILS has shown to reduce this risk of rupture especially when the ovarian cyst is large.7
Because SBT is more common than other types of GYN cancers in reproductive aged women and is associated with infertility, fertility sparing surgery (FSS) is often chosen.4 FSS, sometimes referred to as conservative surgery, involves preservation of the uterus and at least one ovary. Unfortunately, fertility after conservative treatment SBT is difficult to assess given the multivariable nature and long follow up required. Not all women attempt to conceive and some pathologies may have existed before treatment. Recently more studies have attempted to quantify the success of fertility after conservative surgery.12 This should be considered and discussed with the patient prior to treatment and may include fertility counseling.
Recently, Lee et al. compared two subtypes of fertility sparing surgery for borderline ovarian tumors and found they had a high rate of pregnancy after surgery where 79.2 and 87.5% respectively of the patients were able to conceive and most carried to term.13 Similar results were published by Song in 2011 and Park in 2009 with fertility rates upwards of 70% but in 2011 Kanat Pektas had a rate of only 52%.14–16 A meta analysis by Vasconcelos in 2015 revealed an overall rate of approximately 50-60% and was consistent with a previous meta analysis done by Darais in 2011.17,18 Even though published results vary, it is important to note when considering conservative surgery that many studies have show it does not increase mortality even if recurrence is higher.18,19
According to a retrospective review of borderline ovarian tumors (BOT) from 1979 to 2008 consisting of 266 patients, only 8.6% of the entire cohort developed recurrent disease.20 In another retrospective study of borderline ovarian tumors from 1984 to 2008 involving of 233 patients, 21 patients developed recurrent disease.21 There are multiple prognostic factors that influence recurrence rates in patients with borderline ovarian tumors, such as age at diagnosis, baseline CA-125, stage, and histology. Progression-free survival (PFS), defined as the time of diagnosis to the time of recurrence, death or last follow up, is used to assess these prognostic factors. Those with an elevated baseline of CA-125 had a 87% PFS at 3 years and those with a normal baseline of CA-125 had a 97% PFS at 3 years.20, 22, 23 Another important prognostic factor for serous BOTs is the presence of micropapillary patterns, which has a 3 year PFS rate of 76%, compared to the absence of micropapillary patterns, which has a 3 year PFS of 94%.20,24–26
Ovarian cancer is the leading cause of gynecological cancers in women in the US and early diagnosis/staging is important in predicting progression-free survival. Instead of laparotomy, we utilized single-incision laparoscopic surgery to drain the patient’s pelvic effusion and excise the right ovary and fallopian tube, which revealed an ovarian borderline serous tumor. This technique was conservative and fertility-sparing because it left the uterus, left ovary and left fallopian tube. Even though mixed results have been recorded on the rate of fertility after conservative surgery there is conclusive evidence to suggest that conservative surgery does not effect the mortality rate of patients with SBT.15 Even if recurrence does occur frequently with FSS they are easily treated again. Therefore with no effect on mortality, conservative surgery allows for the opportunity of conceiving after SBT.
The authors have self-financed.
Authors did not report any potential conflicts of interests.

©2018 Rezai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.