MOJ
eISSN: 2574-8130


Research Article Volume 1 Issue 5
1Department of Radionuclide Diagnostics, Medical Radiological Research Centre, Russia
2Laboratory of Feinberg School of Medicine, Northwestern University, USA
Correspondence: Vladimir Zaichick, Department of Radionuclide Diagnostics, Medical Radiological Research Centre, Russia, Tel +7 (48439) 60289, Fax +7 (495) 956 1440
Received: April 19, 2017 | Published: July 21, 2017
Citation: Zaichick V, Zaichick S. Age-related changes of some trace element contents in intact thyroid of males investigated by energy dispersive x-ray fluorescent analysis. MOJ Gerontol Ger. 2017;1(5):133-140. DOI: 10.15406/mojgg.2017.01.00028
A prevalence of thyroid dysfunction is higher in the elderly as compared to the younger population. An excess or deficiency of trace element contents in thyroid play important role in goitro- and carcinogenesis of gland. The variation with age of the mass fraction of six trace elements (Br, Cu, Fe, Rb, Sr, and Zn) in intact (normal) thyroid of 71 males (mean age 37.3 years, range 2.0-80) was investigated by 109Cd radionuclide-induced energy dispersive X-ray fluorescent analysis. Mean values ± standard error of mean for mass fractions (mg/kg, on dry-mass basis) of the trace elements studied were: Br 10.8±1.3, Cu 4.25±0.20, Fe 221±13, Rb 10.1±0.89, Sr 4.52±0.43, and Zn 122±5. This work revealed that there is a significant tendency for an increase in Zn mass fraction in normal male thyroid from age 36 years to the eight decade. Moreover, a great disturbance of intrathyroidal trace element relationships with increasing age was found. Therefore, a goitrogenic and carcinogenic effect of excessive Zn level in the thyroid of old males and of disturbance in intrathyroidal trace element relationships with increasing age may be assumed.
Keywords: Thyroid; Trace elements; Age-related changes; Energy dispersive X-ray; Fluorescent analysis
109Cd EDXRF: 109Cd Radionuclide-Induced Energy-Dispersive X-Ray Fluorescence Analysis; CRM/SRM: Certified/Standard Reference Materials; IAEA: International Atomic Energy Agency
The endocrine organs, including the thyroid gland, undergo important functional changes during aging and a prevalence of thyroid dysfunction is higher in the elderly as compared to the younger population.1,2 Advancing age is known to influence the formation of adenomatous goiter and thyroid cancer.3 The prevalence of thyroid nodules is increased in the elderly, reaching a frequency of nearly 50% by the age of 65.4 Both prevalence and aggressiveness of thyroid cancer increase with age.2 Women are affected by thyroid nodule and cancer two to five times more often than men, but in age over 65 years a prevalence of thyroid cancer is higher in men.2-5
Aging is a complex process involving biochemical and morphologic changes in single cells, in organs, and in the whole organism. One of the most generally accepted explanations of how aging occurs at the molecular level is the oxidative stress hypothesis.6 Reactive oxygen species (ROS) are widely considered to be a causal factor not only in aging but in a number of pathological conditions, including carcinogenesis. Aging, considered as an impairment of body functions over time, caused by the accumulation of molecular damage in DNA, proteins and lipids, is also characterized by an increase in intracellular oxidative stress due to the progressive decrease of the intracellular ROS scavenging.7 Oxidative damage to cellular macromolecules which induce cancer can also arise through overproduction of ROS and faulty antioxidant and/or DNA repair mechanisms.8 Overproduction of ROS is associated with inflammation, radiation, and some other factors, including overload of some trace elements, in both blood and certain tissues, or deficiency of other trace elements with antioxidant properties.9-15 Studies have shown that the imbalance in the composition of trace elements may cause different types of pathology. The importance of appropriate levels of many trace elements is indisputable, due to their beneficial roles when in specific concentration ranges, while on the other hand they can cause toxic effects with excessively high or low concentrations.12
In our previous studies16-24 the high mass fraction of iodine and some other trace element were observed in intact human thyroid gland when compared with their levels in non-thyroid soft tissues of the human body. However, some questions about the age-dependence of trace element mass fraction in thyroid of adult and, particularly, elderly females still remain unanswered. One valuable way to elucidate the situation is to compare the mass fractions of trace elements in young adult (the control group) with those in older adult and geriatric thyroid. The findings of the excess or deficiency of trace element contents in thyroid and the perturbations of their relative proportions in glands of adult and elderly males, may give an indication of their role in a higher prevalence of thyroid cancer in the elderly males.
The reliable data on trace element mass fractions in normal geriatric thyroid is apparently extremely limited. There are many studies regarding trace element content in human thyroid, using chemical techniques and instrumental methods.25-30 However, the majority of these data are based on measurements of processed tissue and in many studies tissue samples are ashed before analysis. In other cases, thyroid samples are treated with solvents (distilled water, ethanol etc) and then are dried at a high temperature for many hours. There is evidence that certain quantities of trace elements are lost as a result of such treatment.31-33 Moreover, only a few of these studies employed quality control using certified/standard reference materials (CRM/SRM) for determination of the trace element mass fractions.
This work had three aims. The primary purpose of this study was to determine reliable values for the bromine (Br), coper (Cu), iron (Fe), rubidium (Rb), strontium (Sr), and zinc (Zn) mass fractions in the normal (intact) thyroid of subjects ranging from children to elderly males using 109Cd radionuclide-induced energy-dispersive X-ray fluorescence analysis (109Cd EDXRF). The second aim was to compare the Br, Cu, Fe, Rb, Sr, and Zn mass fractions in thyroid gland of age group 2 (adults and elderly persons aged 36 to 80 years), with those of group 1 (from 2.0 to 35 years), and the final aim was to estimate the inter-correlations of trace elements in normal thyroid of males and their changes with age. All studies were approved by the Ethical Committee of the Medical Radiological Research Center.
Samples of the human thyroid were obtained from randomly selected autopsy specimens of 71 males (European-Caucasian) aged 2.0 to 80 years. All the deceased were citizens of Obninsk and had undergone routine autopsy at the Forensic Medicine Department of City Hospital, Obninsk. Age ranges for subjects were divided into two age groups, with group 1, 2.0-35 years (22.5±1.4 years, M±SEM, n=36) and group 2, 36-80 years (52.4±2.4 years, M±SEM, n=35). These groups were selected to reflect the condition of thyroid tissue in the children, teenagers, young adults and first period of adult life (group 1) and in the second period of adult life as well as in old age (group 2). The available clinical data were reviewed for each subject. None of the subjects had a history of an intersex condition, endocrine disorder, or other chronic disease that could affect the normal development of the thyroid. None of the subjects were receiving medications or used any supplements known to affect thyroid trace element contents. The typical causes of sudden death of most of these subjects included trauma or suicide and also acute illness (cardiac insufficiency, stroke, embolism of pulmonary artery, alcohol poisoning). All right lobes of thyroid glands were divided into two portions using a titanium scalpel.34 One tissue portion was reviewed by an anatomical pathologist while the other was used for the trace element content determination. A histological examination was used to control the age norm conformity as well as the unavailability of microadenomatosis and latent cancer.
After the samples intended for trace element analysis were weighed, they were transferred to -20°C and stored until the day of transportation in the Medical Radiological Research Center, Obninsk, where all samples were freeze-dried and homogenized.35 The pounded sample weighing about 8 mg was applied to the piece of Scotch tape serving as an adhesive fixing backing.36,37
To determine the contents of the elements by comparison with a known standard, aliquots of commercial, chemically pure compounds were used.38,39 The microliter standards were placed on disks made of thin, ash-free filter papers fixed on the Scotch tape pieces and dried in a vacuum. Ten subsamples of the Certified Reference Material (CRM) IAEA H-4 (animal muscle) weighing about 8 mg were analyzed to estimate the precision and accuracy of results. The CRM IAEA H-4 subsamples were prepared in the same way as the samples of dry homogenized thyroid tissue.
The facility for EDXRF included an annular 109Cd source with an activity of 2.56 GBq, Si(Li) detector and portable multichannel analyzer combined with a PC. Its resolution was 270 eV at the 5.9 keV line of 55Fe-source. The duration of the Br, Cu, Fe, Rb, Sr, and Zn measurements was 60 min. The intensity of Kα-line of Br, Cu, Fe, Rb, Sr, and Zn for samples and standards was estimated on calculation basis of the total area of the corresponding photopeak in the spectra. The trace element content was calculated by the relative way of comparing between intensities of Kα-lines for samples and standards. Details of the sample preparation, the facility and method of analysis were presented in our previous publication.36,37
All thyroid samples were prepared in duplicate, and mean values of trace element contents were used in final calculation. Using Microsoft Office Excel, a summary of the statistics, including, arithmetic mean, standard deviation, standard error of mean, minimum and maximum values, median, percentiles with 0.025 and 0.975 levels was calculated for trace element contents. The reliability of difference in the results between two age groups was evaluated by the parametric Student’s t-test and non-parametric Wilcoxon-Mann-Whitney U-test. For the construction of “age - trace element mass fraction” diagrams and the estimation of the Pearson correlation coefficient between different trace elements the Microsoft Office Excel programs were also used.
Table 11 depicts our data for 6 trace elements in ten sub- samples of CRM IAEA H-4 (animal muscle) and the certified values of this material.
Element |
Certified Values |
This Work Results |
||
Mean |
95% Confidence Interval |
Type |
Mean±SD |
|
Br |
4.1 |
3.5-4.7 |
C |
5.0±1.2 |
Cu |
4 |
3.6-4.3 |
C |
3.9±1.1 |
Fe |
49 |
47-51 |
С |
48±9 |
Rb |
18 |
17-20 |
C |
22±4 |
Sr |
0.1 |
- |
N |
<1 |
Zn |
86 |
83-90 |
C |
90±5 |
Table 1 EDXRF data Br, Fe, Rb, Sr, and Zn contents in the IAEA H-4 (animal muscle) reference material compared to certified values (mg/kg, dry mass basis).
Mean: Arithmetical Mean; SD: Standard Deviation; C: Certified Values; N: Non-Certified Values.
Table 2 represents certain statistical parameters (arithmetic mean, standard deviation, standard error of mean, minimal and maximal values, median, percentiles with 0.025 and 0.975 levels) of the Br, Cu, Fe, Rb, Sr, and Zn mass fractions in intact (normal) thyroid of males.
The comparison of our results with published data for the Br, Cu, Fe, Rb, Sr, and Zn contents in the human thyroid is shown in Table 3.
To estimate the effect of age on the trace element contents we examined two age groups, described above (Table 4). Figure 1 shows the individual data sets for the Br, Cu, Fe, Rb, Sr, and Zn mass fraction in all samples of thyroid, and also lines of trend with age. The data of inter-correlation calculations (values of r - coefficient of correlation) including all trace elements identified by us are presented in Table 5.

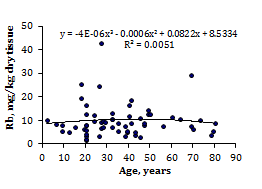
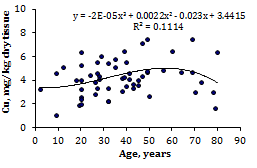
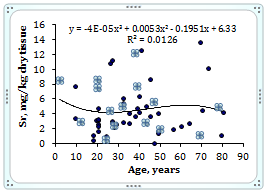
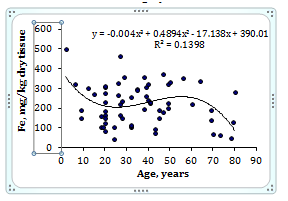
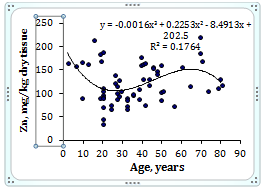
Figure 1 Data sets of individual Br, Cu, Fe, Rb, Sr, and Zn mass fraction values in intact thyroid of males and their trend lines.
Gender |
Element |
Mean |
SD |
SEM |
Min |
Max |
Median |
P 0.025 |
P 0.975 |
Males |
Br |
10.8 |
10 |
1.3 |
1.9 |
54 |
8.05 |
2.33 |
42 |
n=71 |
Cu |
4.25 |
1.48 |
0.2 |
1.1 |
7.5 |
4.15 |
1.78 |
1.39 |
Fe |
221 |
102 |
13 |
47.1 |
502 |
224 |
58.4 |
419 |
|
Rb |
10.1 |
6.96 |
0.89 |
1.8 |
43 |
8.6 |
2.65 |
27.5 |
|
Sr |
4.52 |
3.27 |
0.43 |
0.1 |
14 |
3.55 |
0.443 |
12.4 |
|
Zn |
122 |
41 |
5.2 |
35.4 |
221 |
115 |
57.2 |
201 |
Table 2 Some statistical parameters of Br, Cu, Fe, Rb, Sr, and Zn mass fraction (mg/kg, dry mass basis) in intact thyroid of male
M: Arithmetic Mean; SD: Standard Deviation; SEM: Standard Error of Mean; Min: Minimum Value; Max: Maximum Value; P 0.025 - percentile with 0.025 level, P 0.975 - percentile with 0.975 level.
Element |
Published Data (Reference) |
This Work |
||
Median |
Minimum |
Maximum |
||
of Means |
of Means |
of Means |
||
(n)* |
M or M±SD, (n)** |
M or M±SD, (n)** |
M±SD |
|
Br |
18.1 (11) |
5.12 (44) [25] |
284±44 (14) [26] |
10.8±10.0 |
Cu |
6.1 (57) |
1.42 (120) [27] |
220±22 (10) [28] |
4.25±1.48 |
Fe |
252 (21) |
56 (120) [27] |
2444±700 (14) [26] |
221±102 |
Rb |
12.3 (9) |
≤0.85 (29) [29] |
294±191 (14) [26] |
10.1±7.0 |
Sr |
0.73 (9) |
0.55±0.26 (21) [30] |
46.8±4.8 (4) [28] |
4.52±3.27 |
Zn |
118 (51) |
32 (120) [27] |
820±204 (14) [26] |
122±41 |
Table 3 Median, minimum and maximum value of means Br, Cu, Fe, Rb, Sr, and Zn contents in normal thyroid according to data from the literature in comparison with our results (mg/kg, dry mass basis).
M: Arithmetic Mean; SD: Standard Deviation; (N)*: Number of All References; (N)**: Number of Samples.
Element |
Male Thyroid Tissue |
Ratio |
|||
AG1 2.0-35 Years N=36 |
AG2 36-80 Years N=35 |
T-Test P£ |
U-Test P |
AG2 To AG1 |
|
Br |
8.79±1.53 |
12.6±2.0 |
0.14 |
>0.05 |
1.43 |
Cu |
3.93±0.27 |
4.58±0.28 |
0.098 |
>0.05 |
1.17 |
Fe |
222±18 |
221±18 |
0.963 |
>0.05 |
1 |
Rb |
10.4±1.4 |
9.65±1.09 |
0.682 |
>0.05 |
0.93 |
Sr |
4.15±0.51 |
4.94±0.71 |
0.273 |
>0.05 |
1.19 |
Zn |
112±7 |
135±7 |
0.024 |
£ . 1 |
1.21 |
Table 4 Differences between mean values (M±SEM) of Br, Cu, Fe, Rb, Sr, and Zn mass fraction (mg/kg, dry mass basis) in normal male thyroid of two age groups (AG).
M: Arithmetic Mean; SEM: Standard Error of Mean;T-Test: Student’s T-Test U-Test Wilcoxon-Mann Whitney U-Test Sstatistically Significant Values Are in Bold.
Element |
Br |
Cu |
Fe |
Rb |
Sr |
Zn |
2-35 years |
||||||
Br |
1 |
-0.206 |
0.059 |
0.148 |
0.380a |
0.518b |
Cu |
-0.206 |
1 |
0.420a |
0.364a |
-0.349a |
-0.102 |
Fe |
0.059 |
0.420a |
1 |
0.254 |
0.312a |
0.291a |
Rb |
0.148 |
0.364a |
0.254 |
1 |
-0.028 |
0.067 |
Sr |
0.380a |
-0.349a |
0.312a |
-0.028 |
1 |
0.335a |
Zn |
0.518b |
-0.102 |
0.291a |
0.067 |
0.335a |
1 |
36-80 years |
||||||
Br |
1 |
0.398a |
0.241 |
0.563b |
0.234 |
0.297 |
Cu |
0.398a |
1 |
0.442a |
0.187 |
-0.058 |
0.399a |
Fe |
0.241 |
0.442a |
1 |
0.09 |
-0.166 |
-0.103 |
Rb |
0.563b |
0.187 |
0.09 |
1 |
0.361a |
0.233 |
Sr |
0.234 |
-0.058 |
-0.166 |
0.361a |
1 |
-0.042 |
Zn |
0.297 |
0.399a |
-0.103 |
0.233 |
-0.042 |
1 |
2-80 years |
||||||
Br |
1 |
0.285a |
0.15 |
0.299a |
0.302a |
0.408b |
Cu |
0.285a |
1 |
0.413b |
0.273a |
-0.147 |
0.218 |
Fe |
0.15 |
0.413b |
1 |
0.197 |
0.072 |
0.133 |
Rb |
0.299a |
0.273a |
0.197 |
1 |
0.112 |
0.098 |
Sr |
0.302a |
-0.147 |
0.072 |
0.112 |
1 |
0.174 |
Zn |
0.408b |
0.218 |
0.133 |
0.098 |
0.174 |
1 |
Table 5 Intercorrelations of the chemical element mass fractions in the intact thyroid of male of three age groups (r - coefficient of correlation).
Sstatistically significant values: a p£0.05, b p£0.01.
A set of existing international CRM prepared from the soft tissues of humans and animals is extremely limited. As is was previously discussed [36] 97% of the self-absorption in the dry sample of human tissue is due to the content of bulk elements: carbon (C), nitrogen (N), oxygen (O), phosphorus (P), and sulfur (S), as well as main electrolytes: calcium (Ca), chlorine (Cl), and sodium (Na). The content of these elements and the mass density of muscle and thyroid in humans are virtually identical [37]. Accordingly, the use of CRM IAEA H-4 as a CRM for the analysis of samples of thyroid tissue can be seen as quite acceptable. Good agreement of the Br, Cu, Fe, Rb, Sr, and Zn contents analyzed by EDXRF with the certified data of CRM IAEA H-4 (Table 1) indicates an acceptable accuracy of the results obtained in the study of trace elements of the thyroid presented in Tables 2-5.
The obtained means for Br, Cu, Fe, Rb, Sr, and Zn mass fraction, as shown in Table 3, agree well with the medians of mean values cited by other researches for the human thyroid, including samples received from persons who died from different non-thyroid diseases.24-30 A number of values for chemical element mass fractions were not expressed on a dry mass basis by the authors of the cited references. However, we calculated these values using published data for water (75%)40 and ash (4.16% on dry mass basis)41 contents in thyroid of adults.
A strongly pronounced tendency of age-related increase in Zn mass fraction was observed in thyroid of males (Table 4). In second group of males the mean Zn mass fraction in thyroids was 1.21 times higher than in thyroids of the first age group. There were no statistically significant differences between the Br, Cu, Fe, Rb, and Sr mass fractions within different age-groups. No published data referring to age-related changes of Br, Cu, Fe, Rb, Sr, and Zn mass fractions in human thyroid was found.
A significant direct correlation between the Zn and Br, Zn and Fe, Zn and Sr, Br and Sr, Cu and Fe, Cu and Ru, Fe and Sr mass fractions as well as an inverse correlation between Cu and Sr mass fractions was seen in male thyroid of the first age group (Table 5). In age group 2 many correlations between trace elements in thyroid found in the age group 1 are no longer evident (Table 5). For example, all correlations between Zn and other trace elements existed in the age from 2 to 35 years disappeared but new correlation Zn-Cu was arisen. Thus, if we accept the levels and relationships of trace element mass fraction in thyroid glands of males in the age range 2 to 35 years as a norm, we have to conclude that after age 35 years the level of Zn and relationships of trace elements in thyroid significantly changed. If some positive correlations between the elements in the group 1 were predictable (e.g., Fe-Cu), the interpretation of other observed relationships and their disturbance with age requires further study for a more complete understanding. No published data referring to inter-correlations of Br, Cu, Fe, Rb, Sr, and Zn mass fractions in human thyroid and age-related changes of these inter-correlations was found.
An age-related increase and excess in metal mass fractions in thyroid tissue, including Zn, may contribute to harmful effects on the gland. There are good reasons for such speculations since many reviews and numerous papers raise the concern about toxicity and tumorigenesis of the metals.42-76 Excessive thyroid Zn level in the older males may be harmful to normal metabolism of cells. 77For example, Zn enhances the activity of telomerase,78 an enzyme thought to be responsible for unlimited cell proliferation.79 Excessive Zn intake has undesirable metabolic effects, such as immune dysfunction80 and impaired antioxidant defense.81 By now much data has been obtained related both to the direct and indirect action of Zn on the DNA polymeric organisation, replication and lesions, and to its vital role for cell division.82,83 Moreover, it is known that Zn is an inhibitor of the Ca-dependent apoptotic endonuclease, which takes part in the internucleosomal fragmentation of DNA. Its consequence is a depression of cell apoptosis.84 Some other ways for Zn to act as a potent anti-apoptotic agent have also been described.85-88 All these facts imply that age-related excessive Zn concentrations in thyroid tissue are probably one of the factors influencing goiter, benign, and malignant tumor of the male thyroid.
All the deceased were citizens of Obninsk. Obninsk is the small nonindustrial city not far from Moscow in unpolluted area. None of those who died a sudden death had suffered from any systematic or chronic disorders before. The normal state of thyroid was confirmed by morphological study. Thus, our data for Br, Cu, Fe, Rb, Sr, and Zn mass fractions in intact thyroid may serve as indicative normal values for males of urban population of the Russian Central European region.
This study has several limitations. Firstly, it would be interesting in future studies to examine the Zn and other trace element levels using similar technique in autopsy specimens of females. Future studies should be also directed toward using other analytical methods which allow extend the list of chemical elements investigated in thyroid tissue.
The 109Cd radionuclide-induced energy-dispersive X-ray fluorescence analysis is a useful analytical tool for the non-destructive determination of trace element content in the thyroid tissue samples. This method allows determine means for Br, Cu, Fe, Rb, Sr, and Zn (6 trace elements). Our data reveal that there is strongly pronounced tendency of increase in Zn mass fraction in the normal thyroid of male during a lifespan. Therefore, a goitrogenic and tumorogenic effect of excessive Zn level in the thyroid of old males and of disturbance in intrathyroidal trace element relationships with increasing age may be assumed.
We are grateful to Dr. Yu. Choporov, Head of the Forensic Medicine Department of City Hospital, Obninsk, for supplying thyroid samples.
There is no conflict of interest in composing this manuscript.

©2017 Zaichick, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.