MOJ
eISSN: 2574-9722


Review Article Volume 10 Issue 1
Department of Endocrinology and Metabolism, University of San Marino, San Marino
Correspondence: Vittorio Emanuele Bianchi, Department of Endocrinology and Metabolism, University of San Marino, San Marino
Received: January 02, 2025 | Published: January 22, 2025
Citation: Bianchi VE. Testosterone and brain aging. MOJ Biol Med. 2025;10(1):1‒8. DOI: 10.15406/mojbm.2025.10.00232
Testosterone is an essential hormone to maintain brain health and function. It also exerts a specific activity on the peripheral nervous system, maintaining skeletal muscle activity. The brain has a wide distribution of androgen receptors (AR) in the cortical area, hippocampus, hypothalamus, telencephalon, and amygdala. AR is also in the brainstem and spinal cord areas associated with sensory functions and in Purkinje cells of the cerebellar cortex. ARs were found on axons and dendrites, evidencing extranuclear activity. Testosterone regulates neuronal growth, differentiation, survival, or death through both genomic and nongenomic signaling pathways. Testosterone is metabolized in other hormones: in DHT acting on the hippocampus and 17β-estradiol, which explicitly affects dendritic arborization in females and males. Furthermore, testosterone stimulates oligodendrocytes and myelin formation, while estrogens stimulate mitochondrial activity, anti-inflammatory effect, and astrocytes protection.
Testosterone improves the survival of human neurons and astrocytes, acting directly on the mitochondrial membrane and inhibiting the reactive oxygen species. Furthermore, it exerts a protective effect on brain function, preventing Alzheimer’s disease, reducing the formation of amyloid β(Aβ) peptides in cortical neurons, and neurotoxicity. Furthermore, testosterone is an effective therapy to restore hippocampal function and related pathology, increasing adult neurogenesis within the dentate gyrus region of the hippocampus through an androgen-dependent pathway. Testosterone stimulates myelin regeneration, representing the primary therapeutic goal in demyelinating diseases. There is evidence that it can be effective in various neurodegenerative diseases, such as Parkinson’, SLA, and multiple sclerosis (MS). In this review, the effect of testosterone on neurons, demyelinating diseases, muscle strength loss, mood, and depression have been investigated.
Testosterone is the primary androgen secreted by Leydig cells in men and ovaries in women at lower quantities. Testosterone is essential in the development of sexual characteristics in men and in maintaining the function of tissue in both sexes for all life, such as muscular development, strength, cardiac and brain function, body air, and sexual activity. Testosterone plasma levels in men decrease with progressive age,1 and in older men as aged ≥70 years, a significant reduction is observed. In me, at the age of 60 years, low testosterone levels were found. However, testosterone replacement therapy remains a topic of debate due to potential adverse effects, including cardiovascular risks and increased hematocrit levels, which require careful consideration. In the elderly, reductions in muscular strength and unfavorable body fat distribution due to low testosterone levels were observed.2 Low testosterone was associated with tiredness and fatigue. Nowadays, among middle-aged and older, the administration of testosterone is considered an anti-aging strategy.3 This article aims to evaluate the effect of testosterone on the central and peripheral nervous system. However, testosterone therapy may cause some adverse effects; in particular, its impact on cardiovascular events and mortality and the increase in hematocrit and hemoglobin levels should be considered. In contrast, physiological replacement therapy may improve physical function, mental wellness, and sexual activity density.4s
Metabolism of testosterone
The effect of testosterone is complex because it acts on the neuron in different manners because it is metabolized in various hormones with distinct activity on tissues. Testosterone is metabolized in dihydrotestosterone (DHT) after the reduction of the 5α-reductase, and in 17β-estradiol after the effect of aromatase.5 DHT, the most potent androgen, is transformed in androstenedione and in 3α and 3β-diol metabolites, which have estrogenic effects. Testosterone and DHT (but all androgens as DHEAS or synthetic steroids) activate the AR that are widely expressed on neurons and stimulates oligodendrocyte, myelin repair, and axon regeneration.6 17β-estradiol and 3α-diol and 3β-diol stimulate mitochondrial function, anti-inflammatory effect, and exerts astrocytes protection.7 See figure 1 Testosterone also has important metabolic effects: it increases insulin activity, reduces central obesity in obese patients with type 2 diabetes,8 and reduces metabolic syndrome.9 Furthermore, many synthetic derivates from testosterone (such as oxandrolone, stanazolol, nandrolone, methenolone, etc.) are the androgen anabolic steroids that activate the AR and ER.
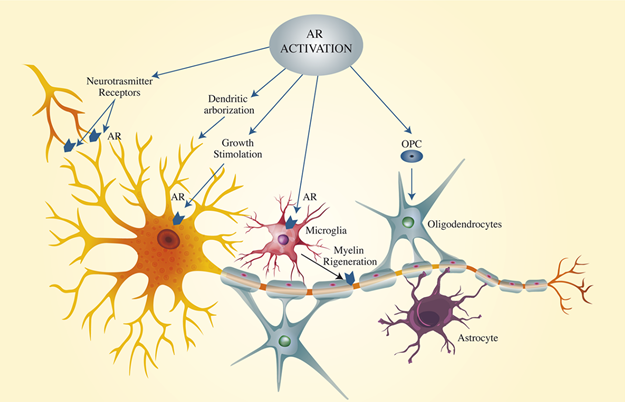
Figure 1 The metabolic way of testosterone. Testosterone, can be transformed in DHT, and 17 β-estradiol DHEAS is produced by adrenal gland. Testosterone, DHT and DHEAS activate AR receptors that simulate oligodendrocyte precursor cells and, consequently, their activation. Oligodendrocytes stimulate myelin formation and axon regeneration. 17 β-estradiol activates the ERs, which increases mitochondrial function, protects the astrocytes, and exerts an anti-inflammatory effect.
Effect of testosterone on the brain
Testosterone and other steroid hormones can cross the blood-brain barrier BBB.10 The effect of testosterone on the brain is due to the extensive-expression in the brain of AR, which was detected in the cortical area, hippocampus, hypothalamus, telencephalon, and amygdala.11 ARs are also expressed in the brainstem and spinal cord areas and the cerebellar cortex.12 Testosterone and its derivatives effectively activate the androgen receptor (AR) on neurons, dendrites, myelin, and astrocytes. Androgens stimulate neuronal growth, differentiation, and death.13 The loss of AR function could contribute to the development of neurodegenerative diseases.14,15 ARs were found on axons and dendrites, evidencing extranuclear activity.16 Figure 2. The brain is an androgen-dependent organ, and its effect its function is largely conditioned throughout life. In the neonatal male, it appears the effect of androgen on the hippocampus and DHT, but not estrogen, exerts a specific effect on dendritic arborization both in females and males17 that is blocked by AR antagonist.18 A neuropathological investigation conducted on postmortem patients of 80 years and older showed lower levels of testosterone in the brain correlated with age, while no changes of 17β-estradiol levels in the brain were observed.19
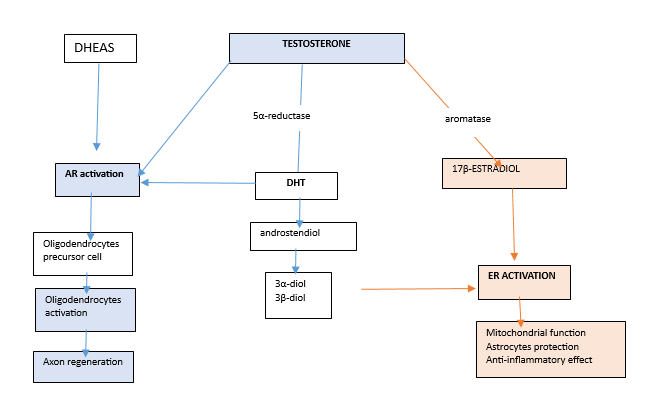
Figure 2 The activation of AR in neurons. The AR distribution is represented. AR are located on the dendrites, on neuron body cell, on oligodendrocytes on microglia. The activation of AR stimulates the neuronal body, dendrites arborization that increase the neural connection, the OPC and microglia that stimulates myelination and axon protection.
In animal models, it was demonstrated that testosterone, through the activation of AR, exerted a protective effect on brain function, preserving Alzheimer’s disease.20,21 In rats, testosterone administration determines an increased secretion of the non-amyloidogenic APP fragment, a reduced formation of Aβ peptides in cortical neurons,22 and reduced neurotoxicity.23,24 This process is mediated by the activation of AR and inhibited by flutamide, an androgen receptor antagonist.21 Testosterone improves the survival of human neurons and astrocytes by stimulating mitochondrial activity and inhibiting reactive oxygen species production,25,26 reactive nitrogen species (NOS) generation,27 and SIRT1 expression.28 These data demonstrate that testosterone is an effective therapy to restore hippocampal function and related pathology.29 Testosterone enhanced synaptic plasticity in rats, scavenging free radicals and increased the number of intact cells and the dendritic spine density in the hippocampal region.21 Neurogenesis occurs in adults, but the functional significance remains to be explained. Sex steroids play a fundamental role in adult neurogenesis. In rodents, testosterone stimulates adult neurogenesis within the hippocampus through an AR pathway.30 The potent regulators effect of androgen on adult neurogenesis in the hippocampus via the AR has also been demonstrated in other than the dentate gyrus.31 Neuroprotection is induced by androgens through the AR activation independently by estrogens.32 Androgens increase cell survival in the dentate gyrus, and this process can be blocked by the administration of flutamide, an AR antagonist.31
The neuroprotective effect of testosterone on the brain has been proposed as a complementary therapeutic intervention in neurodegenerative disease.33 Androgens induce neuroprotection through AR activation independently of estrogens.32 Androgens increase cell survival in the dentate gyrus, and this process can be blocked by the administration of flutamide, an AR antagonist.31 Myelin regeneration is the essential therapeutic goal in demyelinating diseases.34 In Alzheimer’s disease (AD) the regulation of Aβ deposition is regulated by the activation of both AR and ER35 however, testosterone has a direct neuroprotective effect, independently by its conversion into estradiol.24,36 17β-estradiol substantially contributes improving the hippocampal function favouring the cleavage of APP37 and increasing BDNF secretion.38 Estradiol inhibits BACE1 expression39 and induces α-APP secretion by activating the MAPK-signalling pathway independently of ER.40 Testosterone, methyl testosterone, and 17-beta-estradiol reduced neuronal death by 80-90%, and the anti-Aβ effect of testosterone is potentiated by estrogenic levels.41
Testosterone enhanced the synaptic plasticity increasing the number of new cells and the density of dendrites in the hippocampus,21 and reduced the oxidative stress activating SOD and GSH-Px enzymes.42 The neurodegenerative process is strongly sustained by oxidative stress43 and by a deregulation and hypofunction of N-methyl-d-aspartate (NMDA) receptor44,45 as observed in AD.46 The protective effect of testosterone on brain function was demonstrated in animal models preserving from AD.20,21 The proteins in the hippocampus, p-NMDAR1, and p-CaMK II, were correlated with reduced oxidative stress.42 The main action of testosterone on the brain is the effect against oxidative stress,26 and reduction in neuronal death by increasing eNOS activity and SIRT1 expression.28 A low serum testosterone level in men was associated with augmented Aβ deposition in brain tissue, predisposing to the development of AD47-52 while a high testosterone level reduces the onset and development of AD.53 Higher free testosterone levels were associated with lower cerebral Aβ deposits in females. In males, free testosterone was positively related to hippocampal volume and significantly interacted with cognitive status.47,54 Estrogens contribute to the remyelination process in different manners55 in the clinical evaluation of the effects of testosterone should be considered.
Testosterone therapy in patients with AD
Androgen deprivation therapy (SDT) as observed in patients with prostate cancer (PC) demonstrated that men had a a higher risk of cognitive impairment and dementia56 and worsening depression57 confirmed by a recent meta-analysis.58 HowEver, the effect of testosterone administration as a treatment to improve cognition in patients with AD remains still controversial. Many studies evaluated the effect of testosterone therapy in patients with AD21,59-74 which are reported in Table 1. Some studies found positive effects of testosterone therapy on some cognitive function in normal and hypogonadal elderly men,60,62,64,72,75-76 while others had no conclusive results.61,62 The studies conducted on small groups of patients have the risk of many biases. Firstly, the dose of testosterone administration is the most critical point. The testosterone gel has a lower effect than intramuscular injection. Secondly, the plasma levels of other hormones evaluated (as 17β-estradiol, IGF-1) that significantly contribute to brain functions were not regularly evaluated and sometimes without a strong statistical analysis considering the various confounding factors. Importantly, not all screening instruments for the early detection of Alzheimer's disease and cognition function are appropriate to be a promising screening test for memory clinic testing for population screening77 See Table 1.
|
Authors |
Patients |
Age |
Study |
Therapy |
Duration |
Outcomes |
|
Resnick, 2017 |
788 men, Impaired sexual function |
65 |
RCT |
T gel with a dose to maintain the physiological plasma level |
4 years |
No association with improved memory or other cognitive functions. |
|
Wahjoepramono, 2016 |
44 men |
≥50 yrs |
RCT |
T gel 50 mg |
24 weeks and 4 weeks washout, |
Significant improvement in general cognitive functioning. |
|
Huang, 2016 |
308 men with low T. |
60 |
RCT Multicenter study |
T gel 7.5 g of 1% |
36 months |
T administration did not improve cognitive function. |
|
Asih, 2015 |
44, older men |
61 ± 7.7 |
RCT |
transdermal T (50 mg/day) |
24 weeks |
Significant increases in plasma androgens levels. No changes in plasma amyloid-beta. Dementia is not investigated. |
|
Cherrier, 2015 |
351 men community. 37 with MCI and low T |
70.5 ± 8.2 |
RCT |
T gel (50 to 100 mg/day) |
3 months |
Modest improvement in verbal memory and depression symptoms |
|
Borst, 2014 |
60 hypogonadal men |
70.8 |
RCT |
T-enanthate (125 mg/week) |
12 months |
Small improvements in depressive symptoms and visuospatial cognition. |
|
Young, 2010 |
26 young 62 older |
25-35 60-80 |
RCT |
GnRH agonist, T-gel T-gel, 75 mg and 100 mg |
6 weeks |
Free T positively correlated to spatial cognition while estradiol negatively correlated with working memory |
|
Emmelot-vonk,2008 |
237 healthy men with a low T level |
60-80 |
RCT |
T undecenoate 80 mg |
6 months |
Cognitive function and bone mineral density did not change. |
|
Vaughan, 2007 |
65 Healthy men |
RCT |
200 mg of T every 2 weeks with 5 mg of finasteride daily (T+F), or placebo |
36 months |
No clinically significant effect on tests of cognitive function. |
|
|
Maki, 2007 |
15 normal men |
66-87 |
RCT |
T enanthate (200 mg im every other week |
3 months |
Decreased verbal memory and altered relative activity in medial temporal and prefrontal regions. |
|
Charrier, 2007 |
57 eugonadal men |
67 ±11 |
RCT |
T enanthate i.m. 50, 100 or 300 mg/week |
6 weeks |
No significant changes in memory. |
|
LU, 2006 |
16 men with mild AD |
RCT |
T gel (75 mg) |
24 weeks |
T replacement therapy improved the quality of life in AD patients. T had minimal effects on cognition. |
|
|
Haren, 2005 |
76 healthy men |
60 |
RCT |
T undecanoate 80 mg twice daily |
12 months |
Not affect scores on visuospatial tests or mood and quality of life scales |
|
Kenny, 2004 |
11 men with cognitive decline |
80±5 |
RCT |
200 mg every 3 weeks |
12 weeks |
No significant changes in behavior, function, depression, or cognitive performance |
|
Tan, 2003 |
36 men with AD. 10 hypogonadal |
RCT |
Intramuscular T 200 mg every 2 weeks |
12 months |
ADAScog, MMSE, and CDT improved significantly in treated patients |
|
|
O'Connors, 2001 |
30 healthy eugonadal men and 7 hypogonadal men |
RCT |
200 mg of T enanthate i.m. weekly |
8 weeks |
Increased T has a differential effect on cognitive function, inhibiting spatial abilities while improving verbal fluency |
|
|
Cherrier, 2001 |
25 healthy men |
RCT |
T enanthate 100 mg weekly |
6 weeks |
Short-term T administration enhances cognitive function |
|
Table 1 Effect of testosterone therapy on AD and cognitive impairment
RCT, randomized controlled trial; AD, Alzheimer’s disease; T, testosterone; MCI, mild cognitive impairment; ADA Scog=Alzheimer's disease Assessment Scale cognitive subscale; MMSE, mini mental status examination; CDT, clock drawing test; RCT, double-blind placebo-controlled study.
Multiple sclerosis
In Multiple sclerosis (MS), which is an autoimmune inflammatory disease of the CNS and is characterized by neuronal demyelination with subsequent axonal dysfunction and paralysis. Testosterone appears to have a specific indication for the treatment of these alterations. Symptoms of MS range from loss of vision to neuromuscular disorders. Testosterone plays a significant role in the incidence and progression of MS, with a clear predominance in women.78 Estrogen and androgen therapy in MS, has shown encouraging results.79 A study conducted in 10 men with MS with relapse remittent form (RRMS) demonstrated that testosterone exerted a neurotrophic effect on the brain, reducing atrophy and increasing the gray matter in the right frontal cortex. The cortical lesions and brain atrophy are correlated with mental disorders in RRMS.80 These data are of relevant importance for the clinical outcome of these patients. Still, unfortunately, only a few studies on this area have been conducted.
ALS (amyotrophic lateral sclerosis)
ALS is characterized by the primary degeneration of upper (motor cortical) and lower (brainstem and spinal) motor neurons. Muscular atrophy is the consequence of neuron damage. During autopsy, it was found that lateral sclerosis refers to the lateral white matter of the funiculus in the spinal cord (degeneration of the corticospinal tract).81 In a mouse model of ALS, it was found that testosterone compacts the myelin sheet.82,83 Neuronal apoptosis is a complex process that is not yet well understood. Spinal motor neurons degenerate with the reduction of muscle trophic factors, not only when the androgen levels are low but also when the IGF-1 level is significantly low.84 In animal models and humans it was observed the interactions between androgens and IGF-1.85 IGF1 sustains and increases the cellular effect of testosterone.
High doses of testosterone have a negative effect on the endothelial function of the aorta and erectile activity.69 Higher doses of androgen therapy exposure trigger persistent changes in BDNF expression.86 Supraphysiological exposure to androgens exerts neurotoxic effects, increasing the intrinsic apoptotic pathway and alterations in neurite networks. There is a loss of neurite formation and a reduction of the total length of dendrites. Particularly in neurodegenerative diseases such as Alzheimer, there is the need for more robust clinical evidence. DHT seems more effective in the therapy of ALS because it increases the expression of IGF-1 in muscle, exerting myotrophic and neurotrophic effects. DHT treatment reduces the denervation at the neuromuscular junction and motoneuron loss, ameliorating clinical symptoms in ALS, and can be considered a therapy to improve the clinical outcome.87 ALS and its psycho-neuro-endocrinological sequelae should become an area of intensive study in the future.88 Further studies on the effects of androgens in ALS should be explored because they can be of relevant help to the patients.
Effect on muscular strength
The anabolic effect of testosterone is due to increased muscle protein synthesis,89 stimulating satellite cell replication,90 inhibiting muscle protein degradation,91 and increasing neural growth. In elderly men, testosterone administration, after six months of therapy, induced an increase in total lean body mass and muscular strength92 and maximal voluntary strength in a dose-dependent manner but no effect on endurance.93 In aging, sarcopenia is due to fiber and mitochondrial dysfunction. Sarcopenia is associated with loss of muscular strength, physical disability, and reduced quality of life. The nervous tissue change is substantially involved in the lives of elderly people, and the type 2 fast fibers preferentially undergo denervation. In the recovery process of these patients, reinnervation of the musculature is essential.94 In aging sarcopenia, denervation and muscle fiber atrophy are associated and characterized by motor unit loss and skeletal muscle alterations.95 Physical exercise at high intensity stimulates muscle reinnervation in the elderly.96 The association of androgen therapy significantly increases the neurodegenerative process in the skeletal muscles.33 The neuroregenerative process has also been observed in a Charcot-Marie-tooth patient after oxandrolone treatment for three months.97
Connor et al.,98 in a randomized, double-blind, placebo-controlled, crossover study, showed that testosterone associated with exercise compared to the exercise-placebo group for 12 weeks, with a two-week wash-out, significantly increased muscle strength and physical function. Testosterone treatment to reach physiological plasma concentrations in middle-aged and older men can improve lean body mass, whilst exercise training enhances muscular strength.99 particularly in neurodegenerative diseases such as Alzheimer, highlighting the need for more robust clinical evidence.
Effect on mood disorders and depression.
In elderly men with a decline in testosterone levels, the incidence of depression increases100 it also occurs in young men.83 The Endocrine Society Clinical Practice Guideline established hypogonadism criteria requiring two-morning serum testosterone levels below 280–300 ng/dl (9.7–10.4 nmol/L SI units).84 Testosterone is a neuroactive steroid hormone85 because it acts on DOPA receptors. Men with an average age of 64.5 years who have a total testosterone below 200 ng/dl (6.93 nmol/L SI units) compared to eugonadal men had a higher incidence of depressive disorders.101 In the Rancho Bernardo Study, it was found a significant association between increasing severity of major depressive disorder and low circulating levels of total testosterone in men.102 A systematic review with meta-analysis of case-control studies demonstrated that males with depressive disorders had significantly lower plasma testosterone concentrations than healthy controls103 and, of particular interest, inversely associated with levels of bioavailable and free testosterone and dihydrotestosterone,102,104 not always considered in clinical trials. Furthermore, the loss of interest in most activities and low physical energy, and psychomotor retardation contribute to physical inactivity and depression.
An association between hypogonadism and depression has been observed. Observational, cross-sectional, or longitudinal studies reported an inverse relationship of depression scores in men with circulating testosterone levels.105 Men suffering from depression have lower circulating testosterone levels,102,106-107 and men with hypogonadism can manifest depressive symptoms.108 Hover Seidman et al.109 showed that low level of testosterone was more related to dysthimic disorders than depression. Morphologic and functional studies have confirmed the effects of sexual hormones in cerebral regions of interest men have lower circulating testosterone levels.110,111 Barriere et al.110 demonstrated significant sex differences in gray matter concentration at the level of the gonadotropic axis, in the hypothalamus and pituitary but also within the hippocampus and the amygdala of intact animals. However, more recent studies evidenced that severe depressive symptoms do not respond to testosterone therapy.112 Handelsman et al.113 found that testosterone treatment can have minimal efficacy compared with a placebo, showing reasonable safety for up to 2 years. Generic mood elevation does not necessarily signify treatable depression. Depressed patients showed worse sleep than controls, but no significant difference in endogenous hormone levels was found, but the associations between endogenous sex hormones and depressive symptoms were inconclusive.114
The benefits of testosterone replacement therapy in men with major depressive disorder and low testosterone levels in the clinically defined hypogonadal range remain uncertain and require further investigation.115 For Grossmann et al.116 testosterone treatment for 2 years in a group of 1007 patients improved the health-related quality of life, better baseline physical function, greater sense of coherence, and fewer depressive symptoms. The mental health benefit was associated with weight and waist circumference reduction, but testosterone did not affect psychological and physical function or depressive symptoms. In men with minor or more depressive symptoms, testosterone treatment was associated with small but significant improvements in mood and energy. Testosterone substitution can positively influence the quality of life in older hypogonadal men, as has been demonstrated in large placebo-controlled trials.117
The benefits of testosterone replacement therapy in men with major depressive disorder and low testosterone levels in the clinically defined hypogonadal range remain uncertain and require further investigation.
Depression disorders are a complex clinical condition. The DSM-V (Diagnostic and Statistical Manual for Psychiatric Diseases) defined depressive disorders range from dysthymia/minor depression to major depressive disorder118 is more appropriate for this clinical evaluation. Furthermore, polygenic mechanisms are likely to be critical to the biological heterogeneity that influences testosterone-depression interactions. A genetically informed precision medicine approach using genes regulating testosterone levels and AR sensitivity is required.115 The relationship between depressive symptoms and erectile dysfunction in middle-aged men is robust and independent of aging and para-aging confounders.119 The association between depression, testosterone levels, and sexual symptoms in males is difficult to assess due to numerous confounding factors, such as medical conditions, obesity, smoking, alcohol use, diet, and stress. Testosterone and its metabolites act on many cerebral areas and modulate neurotransmission and mood disorders. In patients with severe depression or bipolar, testosterone therapy should be prescribed with caution because it can increase the risk of suicide attempters.120 In conclusion, in hypogonadal men with dysthymic disorders, the therapy with testosterone can improve mood and mild depression, but in cases of severe depression, it should be used with caution. However, testosterone therapy has been associated with adverse cardiovascular events, such as increased risk of thrombosis, as well as psychiatric effects like mood swings and potential exacerbation of manic episodes in bipolar patients. These risks should be carefully weighed against its benefits in neurodegenerative and muscular conditions.
Testosterone significantly affects the brain and peripheral nervous system, maintaining neuronal health and functions. The action on the neurons is characterized by the presence of AR on the neuron body, dendrites, and axonal myelin. ARs are expressed in cortical area, hippocampus, hypothalamus, telencephalon, and amygdala, as well as in Purkinje cells of the cerebellar cortex. The neurodegenerative process is strongly sustained by oxidative stress in the brainstem and spinal cord areas associated with sensory functions. Testosterone and estradiol exert a strong anti-oxidative effect. Testosterone regulates neuronal growth, differentiation, and survival and stimulates oligodendrocytes, myelin repair, and axon regeneration. Myelin regeneration is the main objective of preventing and treating neurodegenerative diseases such as ALS and MS. Furthermore, testosterone inhibits the Aβ formation, exerting a protective effect on brain function and preserving Alzheimer’s disease.
Neurogenesis occurs throughout adulthood in select brain regions. Unfortunately, only a few clinical studies investigating the effect of androgen on neurodegenerative disease have been conducted, so it becomes hard to draw significant conclusions. In aging, loss of muscular strength and physical disability are associated with denervation and muscle fiber atrophy, and the therapy with androgen is essential to stimulate the recovery process of reinnervation in the musculature of these debilitated patients. The use of testosterone replacement therapy is suggested in men with mood disorders with low plasma androgen levels. Still, the sole purpose of improving major depressive symptoms is not recommended, according to current evidence. Moreover, in patients with bipolar disorder, testosterone therapy should be carefully evaluated because testosterone treatment may increase the risk of manic episodes and suicide attempts. In conclusion, testosterone and its derivatives exert many beneficial effects on brain and neuronal function, and they should be considered more in clinical practice.
None.
The authors declare that there are no conflicts of interest.

©2025 Bianchi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.