MOJ
eISSN: 2576-4519


Research Article Volume 7 Issue 1
1Da Nang University of Medical Technology and Pharmacy, Vietnam
2University of Sciences, Hue University, Vietnam
Correspondence: Tran Thuc Binh, Department of Chemistry, University of Sciences, Hue University, Hue 530000, Vietnam, Tel 84905382006
Received: May 20, 2023 | Published: May 29, 2023
Citation: Luu ND, Duy TD, Binh TT. Simultaneous determination of paracetamol and codeine phosphate in pharmaceuticals using molecular absorption spectroscopy and classical least squares method. MOJ App Bio Biomech. 2023;7(1):41-46. DOI: 10.15406/mojabb.2023.07.00174
In this article, a combined method of molecular absorption spectroscopy and classical least squares is used to simultaneously determine paracetamol (PAR) and codeine phosphate (COP) in pharmaceutical samples without the need for prior separation or extraction steps. The absorption spectra of standard solutions and samples were measured in the wavelength range of 210 to 290 nm with a step size of 0.5 nm. The concentrations of PAR and COP in the sample solutions were calculated using a self-made program written in Microsoft Excel 2016 and Visual Basic for Applications (VBA). The reliability of the method was verified through the accuracy and reproducibility of the measurements when analyzing PAR and COP in Effer-Paralmax® Codeine tablets, and comparing the average values of their concentrations in the samples with the standard HPLC method.
Combining chemometrics with spectroscopic methods using full-spectrum data and various algorithms, statistics, and computers has been studied to simultaneously determine constituents in overlapping spectra mixtures. The advantages of these methods include a simple analysis process that does not require the separation or extraction of compounds from each other, and rapid execution that saves time, chemicals, and utilizes all data for calculations, resulting in increased accuracy in determination. These methods include classical least squares (CLS), partial least squares (PLS), principal component analysis (PCA), artificial neural networks (ANN), Kalman filtering, etc. Some authors have studied the application of these methods for analyzing real objects.1–8
In this paper, we investigate the development of an analysis procedure for simultaneous determination of Paracetamol (PAR) and Codeine phosphate (COP) in Effer-Paralmax® Codeine effervescent tablets that are currently circulating in the Vietnamese market. PAR, with a molecular formula of C8H9NO2, M = 151.17 g/mol, is a metabolite with activity of phenacetin, a pain-relieving and antipyretic medication that is most effective in reducing pain, reducing body temperature in feverish patients by acting on the lower hypothalamus, dissipating heat by dilating blood vessels and increasing peripheral blood flow. COP, with a molecular formula of C18H24PNO7, M = 397.00 g/mol, is a phenanthrene derivative, also known as methylmorphine, that has pharmacological effects of pain relief, cough suppression, and excellent antidiarrheal and neuropathic pain management. Effer-Paralmax Codein tablets combine two main components, PAR and COP, which are used to treat mild to moderate pain, fever reduction, and gastric irritation.9 To analyze these components, different individual analytical methods such as spectroscopic methods,10–12 and HPLC methods13 can be used. The individual analytical procedures require much time and complexity, and HPLC method has a significant advantage but a high cost. According to,10–12 the absorption spectra of PAR and COP overlap, so we chose the UV-VIS molecular absorption spectroscopy method with full-spectrum data and CLS algorithm to simultaneously determine the amount of Paracetamol and Codeine phosphate in pharmaceuticals. The proposed method opens up the possibility of fast, cost-effective analysis that can be applied in practical analysis and pharmaceutical testing.
Equipment and chemicals
Equipment
Chemicals
Analysis method
For determination of PAR and COP simultaneously in a mixed solution, we used the UV-VIS molecular absorption spectroscopy method with a full-spectrum combined with the classic least squares method (CLS) and a calculation program written in Microsoft Excel 2016 and Visual Basic for Applications (VBA). The essence of the method is clearly presented in.8 The process of measuring and calculating the concentration of PAR and COP is as follows:
Step 1: Prepare standard solutions of each component to be determined and their mixed solutions.
Step 2: Scan the solutions in the appropriate wavelength range on the UV-Vis spectrophotometer, save and retrieve the measured data (Export data) in "*.CSV" file format.
Step 3: Run the CLS-Excel program8 to calculate the concentration of components in the mixed solution.
Statistical quantities
Relative error
The relative error between the determined concentration and the prepared concentration (RE %) is calculated as:
(1)
Where C is the determined concentration (µg/mL) and C0 is the known standard solution concentration (µg/mL).
Repeatability
Repeatability is evaluated by the relative standard deviation value RSD%:
(2)
Here, SD is the standard deviation and Cmean is the average concentration after n measurements (µg/mL).
For internal laboratory quality control, the repeatability of the method is achieved when the RSD% values obtained are less than
(3)
where C is the concentration expressed as a fraction. (for example, C= 5 µg/mL = 5.10-6)
Method accuracy
The recovery of the method is calculated based on the standard addition method using the following formula:
(4)
where C2 (µg/mL) is the determined concentration after adding the standard, C1 (µg/mL) is the determined concentration before adding the standard, and Cadd (µg/mL) is the concentration of the added standard.
According to,14 to determine the accuracy of the method, the same sample can be analyzed repeatedly using both the research method and the standard method, and the two average sample values can be compared using the student's t-test.
(5)
Where: texp: experimental student's t-value, : the average values of method A and B; : numbers of experiment of method A and B. Compare the value of texp with the value of t(α, ν), in which α is the chosen significance level, ν is degrees of freedom of two methods. If texp < t(α,ν), then the difference in the average values of the two methods is not significant.
Accuracy and precision of the analytical method
Standard working solutions of PAR 25 μg/mL and COP 25 μg/mL were added to 12 volumetric flasks (VF) numbered from 1 to 12, each containing 25 mL, and the volume was adjusted to the mark with H2O-ACN solvent mixture (9:1, V/V). In VF 13 containing 25 mL, 13.5 mL of PAR standard solution of 50 μg/mL and 3.0 mL of COP standard solution of 25 μg/mL were added and diluted to the mark with the solvent mixture. The solutions were scanned in the wavelength range of 210 – 290 nm with a step size of 0.5 nm. The preparation and measurement were repeated 3 times. The absorption spectra of the solutions are shown in Figure 1. The concentration of PAR and COP in the mixed solutions were calculated using the CLS-Excel program, and the relative error RE% and relative standard deviation RSD% of the analytical results were determined. The analytical results of PAR and COP in the prepared mixed solutions in the laboratory along with the corresponding statistical parameters are presented in Table 1.
|
Sample N0 |
Conc. ratio (mg/mL) of PAR/COP |
Meas. time |
PAR |
COP |
||||
|
CPAR (mg/mL) |
RE (%) |
Statistical quantities |
CCOP (mg/mL) |
RE (%) |
Statistical quantities |
|||
|
M1 |
19:1 |
1 |
18.961 |
-0.21 |
Ctb = 19.019 RSD(%) = 0.267 1/2RSDH = 5.136 REtb(%) = 0.10 |
0.988 |
-1.20 |
Ctb = 0.999 RSD(%)= 1.630 1/2RSDH = 8.000 REtb(%)= -0.07 |
|
2 |
19.056 |
0.29 |
0.992 |
-0.80 |
||||
|
3 |
19.040 |
0.21 |
1.018 |
1.80 |
||||
|
M2 |
18:2 |
1 |
18.077 |
0.43 |
Ctb = 17.991 RSD(%)= 0.414 1/2RSDH = 5.178 REtb(%)= -0.05 |
1.975 |
-1.25 |
Ctb = 1.997 RSD(%)= 0.996 1/2RSDH = 7.207 REtb(%)= -0.13 |
|
2 |
17.941 |
-0.33 |
2.004 |
0.20 |
||||
|
3 |
17.956 |
-0.24 |
2.013 |
0.65 |
||||
|
M3 |
17:3 |
1 |
17.070 |
0.41 |
Ctb = 17.075 RSD(%)= 0.029 1/2RSDH = 5.223 REtb(%)= 0.44 |
2.970 |
-1.00 |
Ctb = 2.990 RSD(%)= 0.595 1/2RSDH = 6.781 REtb(%)= -0.33 |
|
2 |
17.080 |
0.47 |
2.996 |
-0.13 |
||||
|
3 |
17.076 |
0.45 |
3.004 |
0.13 |
||||
|
M4 |
16:4 |
1 |
15.998 |
-0.01 |
Ctb = 16.008 RSD(%)= 0.097 1/2RSDH = 5.271 REtb (%)= 0.05 |
3.951 |
-1.23 |
Ctb = 3.985 RSD(%)= 0.743 1/2RSDH = 6.493 REtb(%)= -0.38 |
|
2 |
16.000 |
0.00 |
4.005 |
0.12 |
||||
|
3 |
16.026 |
0.16 |
3.999 |
-0.02 |
||||
|
M5 |
15:5 |
1 |
15.027 |
0.18 |
Ctb = 15.027 RSD(%)= 0.010 1/2RSDH = 5.322 REtb(%)= 0.18 |
4.978 |
-0.44 |
Ctb = 5.013 RSD(%)= 0.612 1/2RSDH = 6.279 REtb(%)= 0.27 |
|
2 |
15.026 |
0.17 |
5.028 |
0.56 |
||||
|
3 |
15.029 |
0.19 |
5.034 |
0.68 |
||||
|
M6 |
14:6 |
1 |
14.123 |
0.88 |
Ctb = 14.128 RSD(%)= 0.046 1/2RSDH = 5.378 REtb (%) = 0.92 |
6.034 |
0.57 |
Ctb = 6.072 RSD(%)= 0.545 1/2RSDH = 6.109 REtb(%) = 1.20 |
|
2 |
14.135 |
0.98 |
6.085 |
1.42 |
||||
|
3 |
14.125 |
0.89 |
6.096 |
1.60 |
||||
|
M7 |
13:7 |
1 |
13.068 |
0.52 |
Ctb = 12.973 RSD(%)= 0.654 1/2RSDH = 5.438 REtb(%)= -0.21 |
7.082 |
1.17 |
Ctb = 7.078 RSD(%)= 0.196 1/2RSDH = 5.969 REtb(%)= 1.12 |
|
2 |
12.948 |
-0.40 |
7.090 |
1.29 |
||||
|
3 |
12.904 |
-0.74 |
7.063 |
0.90 |
||||
|
M8 |
12:8 |
1 |
12.029 |
0.24 |
Ctb = 11.953 RSD(%)= 0.551 1/2RSDH = 5.504 REtb (%) = -0.39 |
8.092 |
1.15 |
Ctb = 8.096 RSD(%)= 0.131 1/2RSDH = 5.850 REtb(%)= 1.20 |
|
2 |
11.911 |
-0.74 |
8.088 |
1.10 |
||||
|
3 |
11.919 |
-0.67 |
8.108 |
1.35 |
||||
|
M9 |
11:9 |
1 |
10.984 |
-0.15 |
Ctb = 10.915 RSD(%)= 0.550 1/2RSDH = 5.576 REtb (%) = -0.77 |
9.088 |
0.98 |
Ctb = 9.095 RSD(%)= 0.077 1/2RSDH = 5.747 REtb(%)= 1.06 |
|
2 |
10.889 |
-1.01 |
9.102 |
1.13 |
||||
|
3 |
10.873 |
-1.15 |
9.096 |
1.07 |
||||
|
M10 |
10:10 |
1 |
10.039 |
0.39 |
Ctb = 10.042 RSD(%)= 0.023 1/2RSDH = 5.657 REtb (%) = 0.42 |
10.083 |
0.83 |
Ctb = 10.152 RSD(%)= 0.586 1/2RSDH = 5.657 REtb(%)= 1.52 |
|
2 |
10.043 |
0.43 |
10.185 |
1.85 |
||||
|
3 |
10.043 |
0.43 |
10.187 |
1.87 |
||||
|
M11 |
27:3 |
1 |
26.990 |
-0.04 |
Ctb = 26.990 RSD(%) = 0.007 1/2RSDH = 4.871 RE (%) = -0.04 |
2.970 |
-1.00 |
Ctb = 2.998 RSD(%) = 1.01 1/2RSDH = 6.781 RE (%) = -0.08 |
|
2 |
26.988 |
-0.06 |
2.993 |
-0.23 |
||||
|
3 |
26.992 |
-0.03 |
3.030 |
1.00 |
||||
Table 1 Analytical result of PAR and COP in laboratory mixtures by CLS-Excel method with statistical quantities
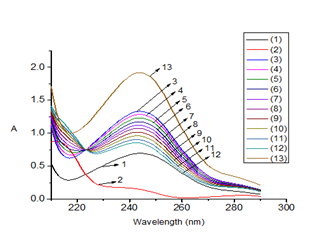
Figure 1 Molecular absorption spectra of PAR10 and COP10 standard solutions and sample solutions at different concentration ratios in laboratory-prepared mixtures:
(1) PAR10 standard solution at 10 µg/mL
(2) COP10 standard solution at 10 µg/mL
(3) M1: Solution containing PAR 19 µg/mL and COP 1 µg/mL
(4) M2: Solution containing PAR 18 µg/mL and COP 2 µg/mL
(5) M3: Solution containing PAR 17 µg/mL and COP 3 µg/mL
(6) M4: Solution containing PAR 16 µg/mL and COP 4 µg/mL
(7) M5: Solution containing PAR 15 µg/mL and COP 5 µg/mL
(8) M6: Solution containing PAR 14 µg/mL and COP 6 µg/mL
(9) M7: Solution containing PAR 13 µg/mL and COP 7 µg/mL
(10) M8: Solution containing PAR 12 µg/mL and COP 8 µg/mL
(11) M9: Solution containing PAR 11 µg/mL and COP 9 µg/mL
(12) M10: Solution containing PAR 10 µg/mL and COP 10 µg/mL
(13) M11: Solution containing PAR 27 µg/mL and COP 3 µg/mL
Table 1 shows that at different concentration ratios of PAR and COP, all RE% values are very small (≤1.6%), therefore, within the concentration range of PAR (10-27 μg/mL) and COP (1-10 μg/mL), with different concentration ratios, the method error for both compounds is small and acceptable. On the other hand, all calculated RSD% values are much smaller than 1/2RSDH, so it can be concluded that the repeatability of the method is very good for laboratory mixed solutions with the investigated concentration ranges and concentration ratios of the compounds given above.
Development of actual sample analysis procedure
Sample preparation
Weigh 20 tablets, determine the average weight of each tablet (Mmean), grind into fine powder and mix well. Weigh an amount of the drug powder equal to the average weight of one tablet on an analytical balance, put it into a 250 mL flask, add about 150 mL of solvent H2O-ACN 9:1 (V/V), then put it in an ultrasonic machine for about 30 minutes, volumetrically adjust with the solvent to the mark (solution 1). Then filter solution 1 through a blue ribbon filter paper and take 10 mL of the filtered solution into a 100 mL volumetric flask and add the solvent to the mark (solution 2), take 10 mL of solution 2 into a 100 mL volumetric flask and add the solvent to the mark (solution 3), shake well to get the sample solution. To compare the results, take 20 tablets (same batch number, manufacturer, and expiration date) to the Testing Center for Drugs, Food and Cosmetics of Thua Thien Hue to analyze by standard HPLC method.
(6)
Where: C (µg/mL): concentration of each substance determined in the sample solution. m: mass of the sample weighed for analysis (mg), Mmean: average weight of one tablet.
Simultaneous quantification of PAR and COP in drug samples
We conducted a determination of the amounts of PAR and COP in Effer-Paralmax® Codein tablets manufactured by BOSTON Vietnam Pharmaceutical Joint-Stock Company, located at No. 43 Street 8, Vietnam-Singapore Industrial Park, Thuan An, Binh Duong, Vietnam, with the label indicating 500 mg PAR and 30 mg COP per tablet. Batch number: 9003, date of manufacture: 25/12/2021, expiration date: 25/12/2024. The average weight of one tablet was M = 3215.0 mg. The sample was processed as described in Section 3.2.1. Accurately weighed 3215.0 mg of the drug powder and processed it into a sample solution. The sample solution was scanned in the wavelength range of 210-290 nm with a step of 0.5 nm. The CLS-Excel program was used to determine the concentrations of PAR and COP in the sample solutions. The amounts of PAR and COP in Effer-Paralmax® Codein were calculated according to equation (6). The absorption spectra of the Effer-Paralmax® Codein drug sample solutions are shown in Figure 2. The results of the analysis of Effer-Paralmax® Codein tablets are presented in Table 2.
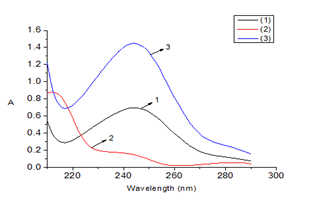
Figure 2 Molecular absorption spectra of standard solutions of PAR and COP, and the sample solution of Effer-Paralmax® Codein.
(1) Standard solution of PAR at 10 µg/mL
(2) Standard solution of COP at 10 µg/mL
(3) Sample solution of Effer-Paralmax® Codein
|
Sample N0 |
H(PAR) (mg/tablet) |
H(COP) (mg/tablet) |
|
M1 |
514.03 |
31.15 |
|
M2 |
523.90 |
30.30 |
|
M3 |
504.93 |
31.83 |
|
Average |
514.28 |
31.09 |
|
Announced |
500.00 |
30.00 |
|
RE% |
2.86 |
3.64 |
|
RSD(%) |
1.845 |
2.458 |
|
*1/2RSDH |
5.075 |
7.739 |
Table 2 Quantities of PAR and COP in Effer-Paralmax® Codein tablets
(*The values of 1/2RSDH were calculated based on the average concentration of the measured sample solutions).
The results presented in Table 2 from the simultaneous quantification of PAR and COP in Effer-Paralmax® Codeine effervescent tablets showed that the analytical method had a relative error (RE%) of less than 5%, which is in accordance with the Vietnamese Pharmacopoeia standard.15 The experimental relative standard deviation (RSD%) was also less than 1/2 of the Horwitz RSD (RSDH), indicating good repeatability of the method.
Reliability of the analytical procedure
To demonstrate the reliability of the analytical procedure, its repeatability and accuracy were evaluated.
Repeatability
The results from Table 2 showed that the method had good repeatability, with RSD% values for both analytes below 2.5% and below 1/2 of RSDH.
Accuracy
The accuracy was determined by calculating the recovery and comparing the results obtained from the study method with those obtained from the standard HPLC method.
Recovery
To determine the recovery, the sample powder mass was weighed using the average mass of one tablet. The first sample did not contain any standards, while the remaining samples contained increasing amounts of both PAR and COP standards. The samples were processed according to the procedure described in 3.2.1 to obtain the sample solution without standards and three sample solutions with added standards. The spectra of the PAR 10 µg/mL and COP 10 µg/mL standard solutions, as well as the spectra of the sample solution without standards (S0) and the sample solutions with added standards (S1, S2, and S3), were recorded. The concentrations of PAR and COP in the sample solutions and the sample solutions with added standards were calculated using the CLS-Excel filter program. The spectra of the standard and sample solutions with added standards are presented in Figure 3, while the concentrations of the added standards and the calculated concentrations of the sample solutions without and with added standards are shown in Table 3. The recovery of the two analyte concentrations in the samples with added standards was calculated using Equation (4).
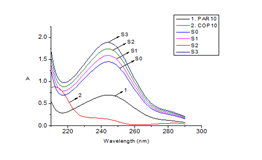
Figure 3 The molecular absorption spectra of standard solutions, sample solutions, and standard-spiked sample solutions.
1. PAR10: standard solution PAR 10 µg/mL; 2. COP10: standard solution COP 10 µg/mL
S0: sample without added; S1: sample added standard the first time (PAR = 2,0 µg/mL, COP = 0,5 µg/mL)
S2: sample added standard the 2sd time (PAR = 4,0 µg/mL, COP = 1,0 µg/mL)
S3: sample added standard 3rd time (PAR = 6,0 µg/mL, COP = 1,5 µg/mL)
|
Sample |
Substance |
C1 (µg/mL) |
C2 (µg/mL) |
Cadd (µg/mL) |
Rev (%) |
|
S1 |
PAR |
20.461 |
22.514 |
2.0 |
102.65 |
|
COP |
1.246 |
1.739 |
0.5 |
98.60 |
|
|
S2 |
PAR |
20.461 |
24.568 |
4.0 |
102.68 |
|
COP |
1.246 |
2.240 |
1.0 |
99.40 |
|
|
S3 |
PAR |
20.461 |
26.550 |
6.0 |
101.48 |
|
COP |
1.246 |
2.730 |
1.5 |
98.93 |
Table 3 Recovery of the method for Effer-Paralmax® Codeine effervescent tablets
From the results of the Rev% recovery calculation presented in Table 3, it can be seen that the recovery of the method is approximately 100% for both substances. This confirms that the analytical method has good accuracy.
Comparison of the analysis results of the research method with HPLC method
To continue the objective evaluation of the accuracy of the current research method, we compared the results of the determination of the amount of substance in the Effer-Paralmax® Codeine drug sample using the analytical method with the results of the standard HPLC method performed by the Drug, Food, and Cosmetics Testing Center of Thua Thien Hue province. We evaluated the results of the two methods statistically.14 The results are presented in Table 4.
|
HPAR (mg/tablet) |
HCOP (mg/tablet) |
||
|
CLS |
HPLC |
CLS |
HPLC |
|
514.03 |
520.10 |
31.15 |
32.87 |
|
523.90 |
527.68 |
30.30 |
32.08 |
|
504.93 |
514.14 |
31.83 |
32.12 |
|
H1(mean)= 514.28 |
H2(mean)= 520.64 |
H1(mean)= 31.09 |
H2(mean)= 32.36 |
|
texp = 0.944 |
texp = 2.478 |
||
|
t(0.05; 4) = 2.78 |
t(0.05; 4) = 2.78 |
||
Table 4 Comparison of the analysis results of PAR and COP content determination in the Effer-Paralmax® Codeine drug sample using the CLS method and the HPLC method
The results obtained in Table 4 show that all texp values are smaller than t(0.05; 4), therefore, the results of the analysis of the two active ingredients PAR and COP in Effer-Paralmax® Codein tablets by the CLS method and the HPLC standard method are statistically equivalent with a significance level of α = 0.05, and it can be said that the analysis results of the CLS method are not different from the results of the standard HPLC method.
With the goal of applying the UV-Vis molecular absorption spectroscopy method combined with the classic least squares (CLS) method to simultaneously determine the content of Paracetamol and Codeine phosphate in Effer-Paralmax® Codein effervescent tablets, we have achieved the following results:
Suitable conditions were studied and selected to simultaneously determine PAR and COP in laboratory mixed solutions containing the two substances with different concentration ratios using the molecular absorption spectroscopy method combined with the CLS method, specifically: Water: Acetonitrile (9:1 v/v) was chosen as the solvent for dissolution; The appropriate wavelength range for spectrum scanning is from 210 nm to 290 nm with a step of 0.5 nm. With different concentration ratios of PAR and COP in the mixed solution, the method errors were less than or equal to 2% for both PAR and COP, and the RSD% values were all small (<1.7). Therefore, the method ensures accuracy and repeatability.
The analytical procedure for the tablet samples containing PAR and COP was established using the UV-Vis molecular absorption spectroscopy method combined with the CLS method, and the reliability of the analysis procedure was demonstrated through the analysis of Effer-Paralmax® Codeine tablets. The results of the analysis drug content had a relative error compared to the label RE% < 5%, which is comply with the quality standards of the Vietnamese Ministry of Health.15 The experimental RSD% was less than 1/2RSDH, so the method has good repeatability. Comparing the results of determining the content of PAR and COP in Effer-Paralmax® Codeine analyzed by the research method and the standard HPLC method showed that the results of the two methods are identical at a significance level of α = 0.05.
None.
The data used to support the finding of this study are available from the corresponding author upon request.
None.
The authors declare that they have no conflicts of interest.

©2023 Luu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.