Journal of
eISSN: 2574-8114


Research Article Volume 8 Issue 3
1Department of Textile Design, National Institute of Fashion Technology, Kannur, India
2Department of Fibres and Textile Processing Technology, Institute of Chemical Technology, Matunga (E), India
3Department of Textile Design, National Institute of Fashion Technology, Patna, India
Correspondence: Pravin P Chavan, Department of Textile Design, National Institute of Fashion Technology, Kannur, India, Tel +91-7977195745
Received: June 30, 2022 | Published: July 18, 2022
Citation: Chavan PP, Teli MD, Pandit P. Sustained release formulation of emulsion and its use for multifunctional cotton fabric. J Textile Eng Fashion Technol. 2022;8(3):86-94. DOI: 10.15406/jteft.2022.08.00305
Today, essential oil-based formulations are used to resist insect bites in humans. Citronella, eucalyptus and lavender oils are known for their mosquito repellent properties. In the present work, an emulsion of chrysanthemum oil was prepared using a binder. The release rate, emulsion stability, and particle size analysis were carried out. The emulsion was applied onto the cotton fabric using the pad-dry-cure technique. The evaluation of the performance properties of the finished material in terms of aroma rating, mosquito repellency on repeated washing and crease recovery angle, as well as bending length and tensile strength, was carried out. The results indicate that near about 100% mosquito repellency can be retained to an acceptable level of 65-75% even after ten washing cycles. The fragrance of the finished fabric enhanced the attractiveness of such a speciality finished material; hence, this technique has promising potential in developing mosquito repellent curtains and home furnishing fabrics.
Keywords: Emulsion, binder, mosquito repellent, cage test, WHO
In today's age of revolution in the world textile industry, every textile sector and every field related to textiles is witnessing the innovations taking place and their beneficial effects. Smart or functional textiles is one field that is presently the fastest growing. Protective textiles are also among such smart technology applications. Protective textiles are the ones which have the functionality of providing protection from mosquitos or insects, bacteria, fungus, heat, cold, etc. Hence, the terms such as antibacterial, mosquito repellent, and heat resistant are used to identify these speciality finishes.1–4 Medical Textile is also one area requiring massive attention as it is directly connected to human beings. The domestic medical textiles and export market has considerable potential.5,6 The fabric prepared to offer protection against insect bites and bacteria also comes in the medical textiles category and is becoming increasingly important for consumers.
The enlightened consumer requires such kind of mosquito repellent textile because diseases originating from mosquitoes affect millions globally every year. The bite of a mosquito can involve anything from contracting malaria to skin irritation. WHO (World Health Organisation) describes dengue fever as the most critical arbovirus which infects thousands of humans in the world.7 In general, the mosquito repellent textile material is prepared by finishing textile products with some mosquito repellent chemicals and protects against mosquitoes that transmit viral infections such as dengue fever (DF), malaria and dengue haemorrhagic fever (DHF), filariasis and chicken-guinea. These diseases are communal severe health issues in tropical regions, especially in Africa and Asia and are transmitted to human beings through mosquito bites only.8 To overcome these diseases, there is a need to use mosquito repellent products. The products used to counter mosquitoes has different degrees of effectiveness.9
Fragrance finishing with prolonged releasing properties for textile material is challenging for the researchers and the manufacturer.10 The best preventive measure for mosquito bites is to evade infested areas that are always not possible or to wear protective clothing and insect repellent.11 Most plants contain compounds that help prevent an attack from phytophagous insects (i.e. plant-eating). These elements fall into numerous classifications: repellents, growth regulators, feeding deterrents, and toxins. Although the significant roles of these compounds are protection against phytophagous insects, some are also efficient against mosquitoes and other insects.12
Chrysanthemum genus (Asteraceae) has been proposed for many years in herbal remedies. It has been used to prevent arthritis, migraine headaches, and anti-inflammatory agents.13 Aerial parts (leaves, flowers and stems) of Chrysanthemum indicum are used to treat hypertensive symptoms, vertigo and many infectious diseases such as fever, colitis, pneumonia, carbuncle and stomatitis. Many researchers have considered the chemical constitutions of essential oil of air-dried Chrysanthemum Indicum flowers and its antibacterial activity has also been established against Streptococcus pneumoniae (S. pneumoniae), Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus).14 A few researchers also identified pyrethrins as an insecticide from Chrysanthemum cinerariae folium flowers. The components extracted using solvents and detected by high-performance liquid chromatography (HPLC) showed that the extract carried Pyrethrin II and Pyrethrin I. it was also found that the flower extract showed an active biological effect in opposition to beetle flour Tribolium Castanum.15 It has been used for an aeon as an aromatherapy effect for treating or mitigating allergies, headaches, inflammation, eye-related diseases and blood hypertension.16 One of the researchers claimed that flavonoids from Chrysanthemum morifolium L. could be used as an agent against AIDS.13 The chrysanthemum oil (CO) was used in composition and can be used on the skin for repelling biting insects, particularly mosquitoes or blood-sucking insects. By combining with other ingredients, the intense fragrance of chrysanthemum oil is screened and the composition has a light and pleasant smell.17 Studied reported that the presence of CO in making a composition gives a freshening effect and has better results on amnesia, listlessness, and other symptoms.18
Chrysanthemum oil (CO) has shown properties to fight against severe and chronic pain because of its anti-inflammatory activity. The safety of CO was also tested and confirmed.19
In the present work, chrysanthemum oil emulsion (COE) was prepared using an acrylic-based binder and applied to cellulosic material like cotton fabric using the pad-dry-cure technique. The emulsion was characterized and the performance properties of the finished fabric were tested to investigate the application potential of chrysanthemum oil emulsion in developing mosquito repellent textile material.
Materials
Chrysanthemum essential oil (Supplied by Siddhi Aroma Chemicals, Mumbai) was used as a mosquito repellent. TATA Mills, Mumbai, India supplied ready for dyeing cotton fabric of 108 GSM. Pidicryl Binder SUN was provided by Pidilite industries ltd, Mumbai. Ethanol, acetic acid were of analytical grade and supplied by S D Fine Chemicals and Ami Chemicals Ltd, Mumbai. The Auxipon NP, a non-ionic detergent, was provided by Auxichem Ltd., Mumbai.
Preparation of emulsion
A ratio of ethanol and distilled water (1:3) was used to prepare oil in water emulsion. In this ethanol-water system, 1ml, 5ml or 10ml of Chrysanthemum oil (CO) as per concentration required were added, to make a total volume of 100ml liquor solution. An acrylate binder Pidicryl SUN for the binding property was added to the solution and the emulsion was prepared using a high-speed homogeniser with 2000-2500rpm for 60mins at 60°C.
Application using the pad-dry-cure method
Padding of fabric was done with 90% expression with Chrysanthemum oil emulsion (COE) and it was followed by drying at 80°C for 2min and then curing at 100°C for 2mins. The treated fabric which is prepared by using an increasing concentration of CO in the emulsion was named; S10- Fabric treated with COE prepared by using 1% CO, S50- Fabric treated with COE composed by using 5% CO, S100- Fabric treated with COE prepared by using 10% CO.
Emulsion stability tests
To understand the kinetic stability of the prepared emulsion, it was kept under observation for 24 hours. The change in droplet size was observed hourly by using the freeze-thaw test. The test was also used to measure the emulsion stability against any change in the temperature. A fixed sample volume was kept under -100C in a freezer during the test to verify any possible sign of separation of oil and water.
Gas Chromatography/Mass spectroscopy (GC/MS)
The study was conducted using GC/MS (EI) on the ITS 40 Finnigan MAT system. Finnian MAT bank and NIST library were used to join the system. The capillary column with an internal diameter of 0.25mm, 30m length and film thickness of 0.25 µm was used for the study. DB-5 (JW). was used as the stationary phase. The study was initiated by keeping the below parameters to detect the components; Temperature was maintained at 50°C for 5minutes and later increased at the rate of 5°C/min till it reached to 300°C and hold at the same temperature for another 5minutes. The carrier gas used was Helium. The sample size was 1 and the split ratio was 1:80.13
Scanning Electron Microscopic (SEM) analysis
The treated fabric was then analysed using a scanning electron microscope (SEM) (ZEISS EVO 50) to see the binding of COE with cotton fabric and compared with the untreated cotton fabric sample. The SEM analysis was conducted by mounting the treated and untreated material on aluminium stubs hard-anodized using electrically conductive carbon tapes in continuation by coating with gold using sputter coater for the 60s.
Particle size analysis
The nanoparticle size analyzer was used to measure the average particle size of the COE and the instrument used was SALD-7500 Shimadzu (Wings SALD II: Version 3.1.1). This instrument works on the principle of Dynamic Light Scattering (DLS).
Attenuated Total Reflection (ATR) Fourier Transform Infra-red (FTIR) Analysis
To study the IR spectra of CO, COE-treated cotton fabric and untreated cotton fabric samples, FTIR spectrometer (i.e. Shimadzu 8400S, Japan) was used. These IR spectra were recorded using ATR module having diamond/ZnSe crystal on FTIR Spectrometer, showing transmittance (%) mode in the range of 4000-800cm-1.
Thermogravimetric Analysis (TGA)
TGA of COE of concentrations 1%, 5% and 10% and CO was conducted and the thermograms were recorded. The instrument used was Shimadzu 60H DTG using the following process. The mechanism operated in the temperature range of 30−500°C under an inert atmosphere of N2 (Nitrogen) gas with the heating rate of 10°C/min. The N2 gas flow rate was maintained at 50mL/minutes.
Mosquito Repellency testing by Cage Test Method
The Mosquito-repellent properties of the untreated and treated samples were evaluated per the standard cage test method. The mosquito rearing cage was prepared of 40×30×30 cm3 dimensions and maintained with the temperature of 25±3°C and 65±5% of relative humidity (RH). Around a hundred mosquitoes were delivered in the cage and allowed to stabilize in the area until further testing. The fabric samples with different concentrations of COE treatment were wrapped around the arm and exposed to the mosquito rearing cage for 30 minutes with the time interval of 2minutes, 5minutes, 10minutes, 20minutes and 30minutes. The mosquitoes' landing behaviour towards the treated and untreated samples at varying time intervals was reported. The mosquito repellency (%) was defined as the percentage reduction in the number of landings when the arm was wrapped with a treated fabric sample, compared to that when the arm was covered with the control (untreated) fabric sample (Figure 1).4
The results were reported according to the below equation:
(1)
Where, C is the number of mosquitoes landing from the control (untreated) fabric sample and T is the number collected from the treated fabric sample.
Test animals
The mosquitoes used for the study were bred in the laboratory. All the egg hatching and breeding processes were carried out in dechlorinated water and the larvae were fed with glucose powder. After coming to the pupa stage mosquitoes were shifted into the jar where they can grow as adult mosquitoes and these adult mosquitoes were fed with 10% sucrose solution. These adult mosquitoes bred in-house were used for testing samples.
Qualitative evaluation of aroma
To carry out the sensory intensity of the fragrance and samples, a Lewi's procedure is followed where a panel of 10 judges was prepared to evaluate. To evaluate the fragrance an insulated booth was prepared to keep the treated fabric on the open desk and the temperature maintained was room temperature.
As per Lewi's procedure, a standard sample is to be given to all judges to rank the process. All the washed and unwashed samples should be packed in an airtight polythene bag to prevent the fragrance release between the evaluation. This also will help to prevent exposure to light and air. The stabilising room was created where samples were placed for 1 hrs to stabilise the evaporation of fragrance before being judged. The sample was evaluated and placed on a flat, hard board on a desk. The judges were asked to use their fingernails to scratch a "×" mark (3cm´3cm) on the sample and take a smell of the sample. The judges were asked to take 3-4 sniffs of the sample and rank them as per the rating scale. They also performed testing for 15 minutes only and after that, they have to take a rest or they can use the smell of strong coffee to clear their nostrils. Judges evaluated the durability of fragrance after several washing cycles and reported their ratings.1
The rating of fragrance was done as per the following rankings;
'+++++᾿ Denotes very strong, '++++᾿ Denotes strong, '+++᾿ Denotes common, '++᾿ Denotes weak, '+᾿ Denotes very weak, '-᾿ denotes not detectable.
Condition for one washing cycle
To study the washing cycles, the ISO 105-C10 standard was used. According to the ISO II test method, a soap solution containing 5 gpl soap was used as washing liquor and the temperature was maintained at 50oC for 45minutes using a 1:50 material to liquor ratio. The instrument used is a wash fastness tester also called a launderometer.
Evaluation of colour change
The cotton fabric treated with microencapsulated citronella oil was subjected to test L*, a* and b* values in terms of CIELAB colour index. All the samples were analysed for these values using Computer Colour Matching System of Spectra flash® SF 300 supplied by Data colour International, USA.
Antibacterial Testing
To study the quantitative evaluation of the antimicrobial activity of COE treated and untreated cotton fabrics against both gram-negative (E. coli) and gram-positive (S. aureus) micro-organisms AATCC 100-2012 standard test method was used. The antimicrobial activities of the treated fabrics were evaluated by the percentage reduction in the number of bacterial colonies formed concerning the untreated control fabric after incubation (at 37±1°C, 24h) was determined. The percentage reduction in bacterial colonies was calculated using the following equation:
(2)
Where R denotes the reduction (%) in the bacterial count; A denotes the number of bacterial colonies counted from the inoculated treated test sample of fabric in the jar which was incubated for 24h of contact period; B denotes the number of bacterial colonies measured from the inoculated untreated test fabric i.e. control fabric in the jar directly after inoculation (at "0" contact time).
Evaluation of physical properties of the fabric
The fabric samples were subjected to determine fabric strength according to the ASTM D 5035(1995) standard. This standard measures the elongation and breaking strength of fabric samples on the Tinius Olsen H5KS universal testing machine (UTM). The stiffness of the fabric samples was evaluated as per the ASTM D 1388(1996) standard; this was the Standard Test Method using the Shirley Stiffness testing (Cantilever principle) instrument.
Particle size analysis of COE
The particle size evaluation of the COE, which is prepared as per different concentrations of CO was evaluated by the mean droplet size of the dispersed phase. This parameter is considered an essential parameter for the measurement of the quality of the emulsion.20 Instrument used is the Shimadzu SALD-7500 Nano, it uses the dynamic light scattering principle in which a laser is passed through the sample and variation in the intensity of the scattered laser is recorded at a particular angle and based on this theory, it can be suggested that the droplet size of the emulsion mentioned in this study is average droplet size. The emulsion was stored in a full bottle at room temperature for 30 days to see the change in droplet size of the emulsion. The results indicate that the emulsion was stable even after 30 days as there was not much change in droplet size (Table 1).
|
Sample |
Size range of microcapsules (µm) |
|||
|
|
Median diameter |
Modal diameter |
Mean value |
Standard deviation |
|
COE 1% |
0.042 |
0.04 |
0.043 |
0.164 |
|
After 30 days |
0.043 |
0.04 |
0.044 |
0.168 |
|
COE 5% |
0.116 |
0.129 |
0.114 |
0.133 |
|
After 30 days |
0.132 |
0.129 |
0.181 |
0.417 |
|
COE 10% |
0.052 |
0.051 |
0.052 |
0.183 |
|
After 30 days |
0.067 |
0.064 |
0.068 |
0.135 |
Table 1 The droplet size of COE
Stability of emulsion
The properties of emulsion like physical stability, rheological behaviour and other properties are generally measured by the dispersed phase droplet size.20 The change in droplet size, prepared over 30 days was studied to analyse the kinetic stability of the oil droplets. The COE was prepared by mechanical stirring at a speed of 2000-2500 rpm and appeared to be a stable emulsion. The emulsion prepared using this method was found with good stability that even after 30 days, no visual phase separation was observed and the change in droplet size of the emulsion also observed at 1-2%. Following physical stability, the thermal stability of the emulsion is also essential for an extensive application. During the thermal stability study, it was found that if the temperature is reduced the interfacial tension increases and it was difficult to hold the droplet to a smaller size because of the higher interfacial tension. The emulsion droplets will start merging with each other to reduce the interfacial energy. The emulsion may destabilize and separate into two different oil and water layers. The freez-thaw test was conducted to study the emulsion prepared from different concentrations of CO and has not shown any changes in the emulsion. Therefore, a significant improvement in the stability of emulsion was observed.
GC/MS analysis of CO
GC–MS analysis of CO was carried out on a Thermo-Fisher Scientific instrument. As per the results shown in Figure 2, it was found that CO contains constituents like α-terpineol (10.93%) and α-pinene (8.48%), Myrcene (0.29%), Limonene (8.11%), beta-caryophyllene (2.03%) and camphor (5.42%). These constituents present in CO are responsible for the treated cotton fabric's mosquito repellent and antibacterial effect.
TGA of CO and COE
Thermograms are essential to the analysis of emulsion formation and with them, it is possible to obtain information about each constituent that forms the emulsion. Figure 3 shows the thermogravimetric curves as a function of temperature. Table 2 summarises the percentage weight loss of CO, COE 1%, COE 5%and COE 10% with temperature and at a specific time. This study was necessary because the prepared emulsion was also applied on cellulosic biomaterial (Cotton), and its release rate was studied as a function of average weight loss.
|
Weight loss (%) |
CO |
COE 1% |
COE 5% |
COE 10% |
|
|
Temperature (ºC) |
Temperature (ºC) |
Temperature (ºC) |
Temperature (ºC) |
|
0 |
26.92 |
25.33 |
27.92 |
26.73 |
|
10 |
76.25 |
42.37 |
44.81 |
43.95 |
|
20 |
89.31 |
49.89 |
53.45 |
51.79 |
|
30 |
113.43 |
55.3 |
59.22 |
57.29 |
|
40 |
121.04 |
60.01 |
64.06 |
61.65 |
|
50 |
137.92 |
63.87 |
68.01 |
65.77 |
|
60 |
145.37 |
67.28 |
71.81 |
69.27 |
|
70 |
148.25 |
70.55 |
75.22 |
72.47 |
|
80 |
155.46 |
73.47 |
78.5 |
77.87 |
|
90 |
193.23 |
80.39 |
91.74 |
179.21 |
|
100 |
218.81 |
379.22 |
397.42 |
389.43 |
Table 2 Weight loss percentage of CO, COE 1%, COE 5% and COE 10% with an increase in temperature
As can be noted by the profile of the thermogravimetric curves, there were distinct zones of mass loss for each component, meaning that the thermal decomposition went through different stages. CO presents mass loss in a single stage, while COE with different concentrations presents mass loss in two steps.
The mass loss of the CO starts at about 27°C and ends at around 218°C. From Table 2 we can say that CO is very thermally stable and we can see only 10% weight loss at a temperature up to 76.25°C and the time taken was 8 mins. It was found that within 31 mins further increase in temperature up to 218°C, CO showed its complete evaporation. Thus, this oil had high volatility, and protection was needed to extend its durability when applied to surfaces.
When weight loss values of different concentrations of COE were compared (Figure 3), two stages of weight loss were observed, in the first stage weight loss percentage of COE was more. The 90% of the weight loss in case of COE 1% and COE 5% was observed when heating was done up to 80°C-91°C within 6 and 7 mins, respectively. For COE 10%, 80% weight loss was observed up to 78°C. Figure 3 indicates that the higher weight loss in the emulsion (COE) took place as compared to that of CO, and it was only because ethanol and water system were used for the emulsion preparation. This solvent system evaporates entirely at less than 100°C. for the Second stage of thermogravimetric curves, we can say that loss was steady and constant after 80°C in case of COE 1% and 91°C in case of COE 5% and the weight loss was complete up to 379°C and 397°C respectively. In the case of COE 10%, the steady curve was observed at 179°C and weight loss entirely up to 389°C. This continuous curve was observed only because of CO and Pidicryl binder SUN which was present in the emulsion. As COE's concentration increases, we can observe a decrease in weight loss percentage. The overall results of the thermal analysis show that although initially, COE had a higher rate of weight loss than CO, as the temperature was progressively increased the stability of CO was increased after emulsion preparation which was the main reason for the preparation of emulsion. It will give a more stable release of an aroma at room temperature.
Release study of emulsion
One of our study's objectives was to understand emulsification's effect on the release rate of the emulsified oil. It was assumed that emulsification should amplify the release rate of the sample as the droplet size decreases. Therefore the release rate study of the different concentrations of COE samples was carried out over 30 days. The particle size analyzer showed the mean droplet diameter (43nm, 114nm and 52nm) of different concentrations of COE when prepared. The graph was plotted between the average weight of the emulsion evaporated (due to natural evaporation) and the time spent. Figure 4 indicates that the rate of evaporation of the smaller droplet size was high as compared to that of larger droplet size because of the higher interfacial area acquired by smaller droplet sizes.21,22 As the smaller droplet of the same volume of emulsified oil has a higher interfacial area, the effective mass transfer rate of the oil-water interface will also increase. This higher interfacial area is responsible for a higher mass transfer rate and accelerated substrate dissolution in the continuous phase and, consequently, a higher release rate. As we see in Figure 4, it is seen that the release rate of 1% COE is higher than that of 10% COE.
FTIR analysis
As per literature, we can say that the main chemical constituents in the CO are lipophilic compounds, such as aliphatic alcohols, aliphatic acids (esters) and micro molecule terpenoids. The Fourier transform infrared spectra (FTIR) shown in Figure 5 were studied to identify the possible biomolecules responsible for mosquito repellent and antibacterial activity of CO and treated cotton fabric. The intense broadband in CO at 3406cm−1 is due to the O−H stretching and at 2931cm−1, 2871cm−1 is due to the C−H stretch. The most substantial peaks appearing in the 3010–2850cm-1 belong to the stretching vibration of C-H of saturated and unsaturated hydrocarbons, indicating many alkanes or alkenes. The FTIR spectrum, in which the peak intensities between 2931.60cm-1 (CH3) and 2871.29cm-1 (CH2) indicate the equivalent content of CH3 and CH2 in CO. Also can be seen on treated cotton fabric at 2894.95cm-1, an extra peak on treated cotton fabric. The peak at 1429cm-1 in the treated cotton fabric indicates C=C stretch. Another characteristic region is near 1700 cm-1 assigned to the functional group absorption of carbonyl, which suggests that there are many carboxylic esters and many carboxylic acids or terpene ketone compounds. The peak at 1728.10cm-1 is assigned to the C=O functional group absorption, also seen on treated cotton fabric at 1731.96cm-1. FTIR spectra clearly showed the presence of α-terpineol, α-pinene, Myrcene, Limonene, β-caryophyllene and camphor in CO, which are the major constituents. This confirms the presence of COE in the fabric and is responsible for giving functional properties to the treated material.23
SEM analysis
The SEM images in Figure 3 show the topography of the samples. Cotton fabric treated with COE showed that the COE was applied on the surface of the fabric and it was uniformly distributed when compared with the image of the untreated cotton sample. This confirms the deposition of emulsion on the surface of the cotton fabric. It is to be noted that as seen in Figure 6, the SEM image of COE-treated cotton fabric, which showed uniform distribution also has a polymeric binder which holds them to the fabric.
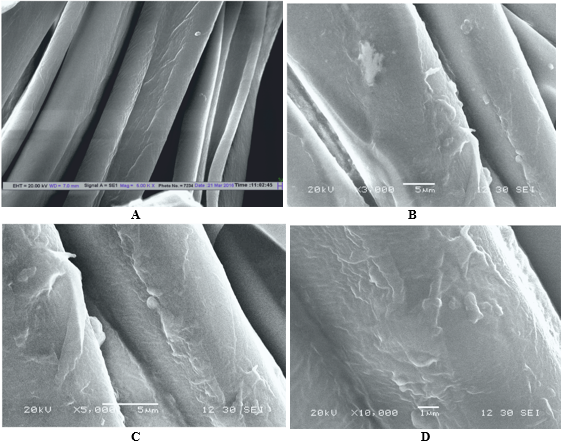
Figure 6 Scanning electron microscope image of cotton fabric. A-Untreated cotton fabric, B- COE treated cotton fabric at 3000X, C- treated cotton fabric at 5000X, D-treated cotton fabric at 10000X.
Mosquito repellency and aroma testing analysis
The results in Tables 3–5 indicate that after 30 min, the fabric samples S10, S50 and S100 showed an almost increased level of mosquito repellency of the order of 94±1% to 98.11% as the concentration of CO was increased. However, after washing the mosquito repellency decreased progressively and it was observed that for 20 washing cycles, the mosquito repellency was found to be maximum i.e. 72.12% in the case of the S10 sample, followed by 60.11% for the S50 sample and 43.75% for S100 sample.
|
No. of washes |
Mosquito repellency (%) |
Mosquito repellency after 30 min. |
Aroma rating |
||||
|
Time (in min) |
|||||||
|
2 |
5 |
10 |
20 |
30 |
|||
|
Unwashed |
100 |
96.16 |
93.64 |
94.23 |
94.182 |
94.18 |
+++++ |
|
5 |
92.52 |
88.28 |
88.16 |
82.66 |
83.29 |
83.29 |
++++ |
|
10 |
83 |
80.82 |
76.5 |
76.24 |
73.17 |
73.17 |
++++ |
|
15 |
62.12 |
68.74 |
65.67 |
62.16 |
58.9 |
58.9 |
+++ |
|
20 |
57.33 |
60.18 |
52.16 |
48.35 |
43.75 |
43.75 |
++ |
Table 3 Effect of sample S10 on percentage mosquito repellency and aroma
‘-’ = not detectable, ‘+’ = very weak, ‘++’= weak, ‘+++’= good (common), ‘++++’= strong, ‘+++++’= very strong.
|
No. of washes |
Mosquito repellency (%) |
Mosquito repellency after 30 min. |
Aroma rating |
||||
|
Time (in min) |
|||||||
|
2 |
5 |
10 |
20 |
30 |
|||
|
Unwashed |
100 |
100 |
96.21 |
95.56 |
95.64 |
95.64 |
+++++ |
|
5 |
100 |
98.28 |
94.34 |
95.14 |
95.74 |
95.74 |
++++ |
|
10 |
89 |
91.17 |
90.4 |
84.41 |
83.16 |
83.16 |
++++ |
|
15 |
85.21 |
87.38 |
77.17 |
75.66 |
72.46 |
72.46 |
+++ |
|
20 |
74.71 |
67.74 |
63.19 |
62.21 |
60.11 |
60.11 |
++ |
Table 4 Effect of sample S50 on percentage mosquito repellency and aroma
‘-’ = not detectable, ‘+’ = very weak, ‘++’= weak, ‘+++’= good (common), ‘++++’= strong, ‘+++++’= very strong
|
No. of washes |
Mosquito repellency (%) |
Mosquito repellency after 30 min. |
Aroma rating |
||||
|
Time (in min) |
|||||||
|
2 |
5 |
10 |
20 |
30 |
|||
|
Unwashed |
99 |
97.27 |
98.12 |
96.15 |
98.11 |
95.64 |
+++++ |
|
5 |
96.23 |
93.64 |
95.15 |
92.31 |
90 |
95.74 |
++++ |
|
10 |
95.79 |
97.27 |
87.13 |
84.61 |
82.21 |
83.16 |
++++ |
|
15 |
95 |
91.68 |
79.23 |
73.34 |
72.69 |
72.46 |
+++ |
|
20 |
91.25 |
87.27 |
77.13 |
74.61 |
72.12 |
60.11 |
++ |
Table 5 Effect of sample S100 on percentage mosquito repellency and aroma
‘-’ = not detectable, ‘+’ = very weak, ‘++’= weak, ‘+++’= good (common), ‘++++’= strong, ‘+++++’= very strong.
It is natural, that for 2 min the mosquito repellency was maximum and as the time of measurement increased the percentage repellency decreased.24 When mosquito repellency was compared against the fabric exposure time it was evident that for the first 2 min of the exposure the repellency of mosquitoes was highest; however, as the time of exposure increased the mosquito repellency decreased.2,25 It is well known that the mosquitoes tend to get adapted to the adverse situation and hence even though at the beginning mosquitoes are repelled to the highest extent when expose to mosquito repellent finish fabric, with time they adapt to such a surrounding and show decreased.26–28
Judges gave the aroma evaluation ratings per the standard procedure; these ratings are shown in Tables 3–5. Initially, in the unwashed form of the sample, it gave a maximum aroma rating of "very strong" (+++++). However, after 5, 10, 15 and 20 washes the aroma ratings progressively decreased and it was "weak" (++) in the case of 20 washes for all samples. After washing cycles, the progressive decrease observed here is understandable due to the washing-off of the emulsion with the increasing number of washing cycles. These results of aroma rating support the effects of mosquito repellency because the whole repellency phenomenon is based on the intensity of the aroma and the constituents present in the essential oil.
The treated fabric was giving mosquito repellent and aroma property because of the constituents present in the CO. Several essential oils commonly appear to be related to the presence of monoterpenoids and sesquiterpenes which have high repellent activity against mosquitoes. According to literature, constituents such as α-pinene, limonene, and camphor are monoterpenes which are common constituents of several essential oils and also exhibits mosquito repellent activity. β-caryophyllene as shown in Figure 7, was identified as one of the constituents of CO, is a strong repellent against mosquitoes and comes into the category of sesquiterpenes. Therefore, the constituents present in CO were responsible for mosquito repellent property and pleasant fragrance, thus giving the fabric the multifunctional property.29
Antibacterial properties
The antibacterial activity of COE-treated cotton fabric with different concentrations of COE was evaluated and the results are expressed in Table 6. The treated fabric showed an increase in antibacterial activity with the rise in the concentration of CO in COE. The antimicrobial activities of COE-treated cotton fabric might be due to the presence of significant concentrations of α-terpineol, α-pinene, Myrcene, Limonene, β-caryophyllene and camphor. These monoterpenes and sesquiterpenes also have antibacterial and antifungal potential.31–32 The more potent activity of the essential oil against bacteria also may be due to the presence of a β-caryophyllene, since the antibacterial properties of caryophyllene were studied and found effective against both gram-positive and gram-negative bacteria.33 The synergistic effects of all the constituents of the major and minor components present in the essential oils should be considered for their antibacterial activity. From the results in Table 6, it can be seen that COE is more effective against S. aureus than E. coli and an increase in the concentration of CO also exhibits a good increase in antibacterial activity even after 20 washes.
|
Sample |
Bacterial reduction (%) |
|||||
|
S10 |
S50 |
S100 |
||||
|
No. of washes |
E.coli |
S. aureus |
E.coli |
S. aureus |
E.coli |
S. aureus |
|
Unwashed |
90.26 |
97.68 |
100 |
100 |
100 |
100 |
|
5 |
75.29 |
78.43 |
91.68 |
93.06 |
93.65 |
97.37 |
|
10 |
64.25 |
67.13 |
80.46 |
86.68 |
87.05 |
91.34 |
|
15 |
62.21 |
63.19 |
70.11 |
78.9 |
85.71 |
88.59 |
|
20 |
48.35 |
52.16 |
57.33 |
65.67 |
71.23 |
77.61 |
Table 6 Antibacterial activity of COE treated fabric
Physical properties of the COE-treated fabric
The results as shown in Figure 8 indicate that the tensile strength of the fabric treated with different concentrations of COE decreased as a result of finishing. This decrease was observed due to the chemical treatment cotton fabric undergoes during finishing. The elongation shown in Figure 9 also showed a similar trend of tensile strength compared to the untreated sample. The bending length values of treated fabric as shown in Figure 10 also showed an increase in the magnitude and a hence slight increase in the stiffness of the fabric, although it was well within the acceptable limit.
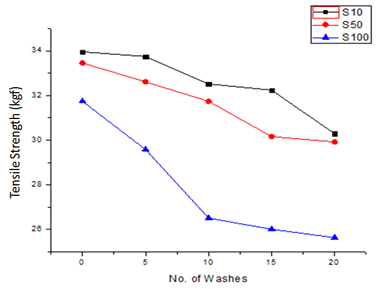
Figure 8 The relationship between tensile strength and number of washes of sample S10, S50 and S100.
The whiteness index of the treated fabric as shown in Tables 7–9 decreased compared to the untreated sample. Still, conversely, the yellowness index on finishing was distinctly higher than that of control samples. These results indicate that after finishing there was a slight but distinct decrease in the whiteness, mainly because of the coverage of the fabric by the COE. The overall increase in the 'b*' values and a decrease in 'a*' values towards the negative side indicate the yellowish-green tonal change responsible for the whiteness index.
|
Samples |
L* |
a* |
b* |
WI |
|
Control |
92.726 |
0.027 |
2.479 |
71.633 |
|
Unwashed |
93.075 |
0.794 |
-2.228 |
91.948 |
|
5 |
92.917 |
0.07 |
0.458 |
81.205 |
|
10 |
92.876 |
-0.147 |
0.942 |
78.483 |
|
15 |
92.833 |
-0.229 |
1.495 |
76.198 |
|
20 |
92.641 |
-0.045 |
1.041 |
77.847 |
Table 7 L*, a*, b* and whiteness index values of S10 samples
|
Samples |
L* |
a* |
b* |
WI |
|
Control |
92.726 |
0.027 |
2.479 |
71.633 |
|
Unwashed |
92.869 |
-0.315 |
1.647 |
78.067 |
|
5 |
92.906 |
-0.452 |
2.03 |
77.383 |
|
10 |
92.875 |
-0.457 |
2.029 |
76.082 |
|
15 |
92.769 |
-0.694 |
2.526 |
73.868 |
|
20 |
92.817 |
-0.418 |
2.091 |
74.18 |
Table 8 L*, a*, b* and whiteness index values of S50 samples
|
Samples |
L* |
a* |
b* |
WI |
|
Control |
92.726 |
0.027 |
2.479 |
71.633 |
|
Unwashed |
90.066 |
-0.658 |
4.526 |
62.343 |
|
5 |
89.718 |
-0.537 |
4.249 |
64.258 |
|
10 |
89.297 |
-0.697 |
3.79 |
64.449 |
|
15 |
89.454 |
-0.771 |
4.073 |
65.468 |
|
20 |
89.121 |
-0.832 |
3.709 |
66.028 |
Table 9 L*, a*, b* and whiteness index values of S100 samples
The COE's which were prepared using acrylic-based binder had nano droplet size and had good stability as it was found that there was no measurable change in particle size even after 30 days of the storage. The release rate of CO decreased during the preparation of emulsion which was necessary for the textile application. Pidicryl binder played the emulsion stabilizer and a binding agent during application onto the Textile. Mosquito repellency was found to be very good even after washing the textile fabric as it exhibited longer protection from mosquitoes than humans. Antibacterial activity was also found to be maximum i.e. more than 50% even after repeated washing cycles. In other words, fabric applied with COE exhibited multifunctional properties and durability in washing.
None.
None.
Author declares that there is no conflict of interest.

©2022 Chavan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.