Journal of
eISSN: 2574-8114


Review Article Volume 10 Issue 6
R&D Chief, Hatin Textile, and Hatin Tex Weaving Companies, DOSAB, 16245, Bursa Province, Turkey
Correspondence: Ömer Firat Turşucular, R&D Chief, Hatin Textile, and Hatin Tex Weaving Companies, DOSAB, 16245, Bursa Province, Turkey
Received: December 19, 2024 | Published: December 30, 2024
Citation: Turşucular OF. Sustainable and eco-friendly Flame Retardants (FR) in polyester fibers: a review. J Textile Eng Fashion Technol. 2024;10(6):244-249. DOI: 10.15406/jteft.2024.10.00395
The growing demand for sustainable solutions for flame retardants (FR’s) in polyester (PET) fibers raises questions about the efficacy and environmental impacts of these products. This theoretical review study included the effective technical parameters, application processes, tests, various physical, chemical, thermal, mechanical, and surface morphology changes of applied FR treatment were technically examined and interpreted. The main purpose of this theoretical review study was to examine the technical aspects of various physical, chemical, thermal, mechanical, and surface morphology changes in polyester (PET), and polyester (PET)/cotton (CO) blended fabric structures, especially in their FR finishing processes, and to guide future technical studies. FR applications have been generally used in military, textile, automotive, and metallic industries. The type of chemical used, concentration (by volume % c), viscosity (Pa.s), molecular weight (Da), pH, temperature (C), time (minutes, or hours), pressure (Pa), low free surface energy, thickness (mm), and environmental conditions (especially are relative humidity (rH), and atmospheric pressure (Po) etc.) of the coating applications have been effective factors on FR applications. The dip-coating, pad-dry cure, sol-gel, layer-by-layer (LbL), and plasma-grafting application methods have been applied for FR applications. The washing (especially are the soap, alcohol, or distilled water), drying, and fixing processes have been applied as post-processes, respectively. LOI test has also been the most important test, too. It must be over 21% ratio. Triazine, formaldehyde, melamine, halogen, phosphorus compound chemicals, ZnB, and silica (Si) nanoparticles have been commonly used as non-sustainable and non-eco-friendly chemicals. Polydopamine (PDA), chitosan (CHI), casein, protein, enzyme, DOPO, APA, β-CD, and boric acid have been commonly used as sustainable and eco-friendly chemicals. They are also FR effectiveness, low smoke, and toxicity, biocompatibility, and cost-efficiency chemicals. In conclusion, the alkaline, or plasma grafting as pre-treatment processes should be applied before applying FR applications. The optimization for FR application should be varied between 8% and 20% (by volume) for the concentration, between 60 °C and 80 °C for the temperature, between 0.5 hours and 3 hours for the time, 7 for pH, 1:10 for flotte ratio with using pad-dry cure, or sol-gel processes. The between 80 °C and 160 °C for temperature for between 3 minutes and 5 minutes for time with distilled water, or ethanol chemicals, which are washing chemicals as drying, and fixing processes, respectively. As the FR concentration increases by volume (% c), mass loss, and burning time increase, and the burning rate decreases. It has also self-charring behavior, too.
Keywords: sustainable, eco-fiendly, polyester (PET) yarns, fabrics, flame retardant (FR), application
In the first main part of this theoretical review study included that the flame retardant (FR) finishing process was examined technically from its purpose, history, applications, mechanism, effective factors, effective technical parameters, LOI values of some polymeric-based materials, chemicals used, application methods, various tests, and standards applied. In the second main part of this theoretical review study was examined technically from sustainable and eco-friendly FR chemicals, and their application processes. In the third main part of this theoretical review study was examined technically from various physical, chemical, thermal, mechanical, and surface morphology changes in PET, and PET/CO blended structures. In the last main part of this theoretical review study was made from various technical interpretations related to various experimental studies, especially from various physical, chemical, thermal, mechanical, and surface morphology changes in structures with PET, and PET/CO blended fabric forms. The growing demand for sustainable solutions for FR in PET fibers raises questions about the efficacy and environmental impacts of these products. The main purpose of this theoretical review study was to examine the technical aspects of various physical, chemical, thermal, mechanical, and surface morphology changes in PET, and PET/CO blended fabric structures, especially in their FR finishing processes. It was explained through theoretical, and experimental studies, and it was aimed to lead technically to various future experimental studies in this scientific field.
Flame Retardant (FR) finishing process
Flame retardant (FR) application is applied to reduce the flammability of flammable textile products and extend the burning time by giving FR properties to polymeric materials.1–5 Improving flame resistance has been an important subject of study in the global textile industry since the Ancient Egyptian Civilization. Starting from the 18th and 19th centuries, modern chemists began to work more intensively on this subject. In fact, the importance of the subject was emphasized in the poem of the US poet Emily Dickinson. In 1893, intending to protect the theaters, the groundbreaking work of the French chemist Joseph Louis Gay-Lussac, who investigated flame retardancy (FR) with the chemicals ammonium polyphosphate (APP), and borate, emerged.2,6,7 In the same year, Scottish chemist M. M. Pattison Muir's technical work titled The Chemistry of Fire was published as an article in the world-famous science journal "The Chemistry". Moreover, in the first half of the 20th century, many scientists working in the field of molecular chemistry worked intensively to explain chemically and improve flame resistance. The development of flame resistance, especially thanks to various studies in the field of polymer chemistry, has enabled the synthesis of new fibers and chemicals (especially are plastic) and the discovery of production methods in the scientific world. The high flammability properties of these especially developed plastic materials (synthetic-based materials) have led scientists to seek innovative solutions to the issue of flame retardancy (FR).1–30 Although some new fibers synthesized by scientists by chemical methods over the years (the examples are meta-aramid (Nomex), and para-aramid (Kevlar), etc.) have inherently FR properties, some other fibers that have been commercialized and widely used in the world (the examples are polyamide (PA), polyethylene (PE), polypropylene (PP), polyurethane (PU), polylactic acid (PLA), polyester (PET), and polyacrylonitrile (PAN) etc.) both the annual production amounts and the fact that they can be produced at incomparably cheaper prices make it more attractive for scientists to study the FR processes of such widely used synthetic-based fibers.6,3–5,14–30 FR applications are military protective clothing, firefighter clothing, workers' clothing in the metal industry, baby clothing, military textiles, sports textiles, construction textiles, geotextiles, automotive, electrical, upholstery seat fabrics, carpet, and home textile products.2,3,7,9,10,12,22,23,26,28 There are criteria such as FR effectiveness, low smoke and toxicity, biocompatibility, cost-efficiency, washing durability, and ease of scaling of FR in FR applications.3,12 FR mechanism, especially in polymeric-based materials, is as follows: the burning of polymeric material occurs through the emergence of flammable volatile substances through decomposition in an atmosphere rich in oxygen (O2). The technical working mechanism of FR application as a solution to the combustion of these polymeric-based materials consists of condensed phase and vapor phase, regardless of the chemical type used. Here, the flame resistance/retardation condensation phase generally involves the formation of a char layer that can stop the spread of flame and protect the material by self-extinguishing, limiting the transfer of heat and oxygen to the polymer. The flame resistance/retardation vapor phase involves terminating the radical chemistry involved in the fire.2,3,6,8–12,16 The flame retardant (FR) mechanism was presented in Figure 1.8
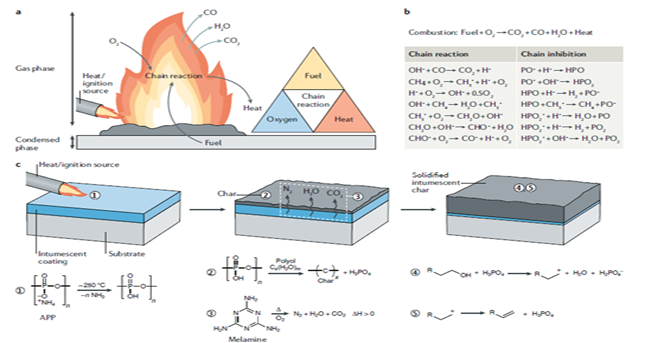
Figure 1 The flame retardant (FR) mechanism.5
FR applications generally occur by adding FR chemicals as low molecular weight (Da) additives to polymeric-based materials and creating strong covalent bonds between the polymeric-based fiber, and FR chemicals.3,6,8 The technical parameters such as heat release rate (PHRR), ignition time (TTI), total heat release (THR), time to peak heat release rate (TTPHRR), fire growth rate (FIGRA), after flame (s), coal length (mm), LOI (%), burning rate (mm/sec), and melt-drip (%), and the burning behavior of the fiber after burning are extremely important and must be determined in FR applications.3–7,10,12,14–30 Textile materials burn rapidly at LOI values of up to 21% by volume. They burn slowly when the LOI is between 21 vol% and 25 vol%. They have FR properties beyond 26 vol% LOI.2 In addition, the FR phenomenon can be determined by standards such as simple ignition tests (BS 5438 and EN ISO 6941). The purpose of this test is to evaluate the flammability of a textile material, more specifically to examine the ignition of a standard gas by applying a flame to the face or bottom edge of a vertically oriented fabric sample.6 Furthermore, the time required to ignite the sample is recorded (seconds (s)). ASTM D2863 standard for LOI test and ASTM D6413 standard for vertical flame test are also widely applied to determine the final result in FR applications.6,26 Moreover, when microparticles (μm) or nanoparticles (nm) are added above the 15% concentration value, the mechanical properties increase significantly.9,19,21 The LOI values of some polymeric-based materials were presented in Table 1 (Figure 2).7
|
Polymer type |
LOI value |
|
Polyethylene (PE) |
17 |
|
Polypropylene (PP) |
17 |
|
Polystyrene (PS) |
18 |
|
Polybutylene terephthalate (PBT) |
18 |
|
Vinyl ester (VE) |
22 |
|
Epoxy (EP) |
25 |
|
Polyethylene terephthalate (PET) |
26 |
|
Polyamide 6 (PA 6) |
26 |
|
Polyamide 6.6 (PA 6.6) |
26 |
|
Polycarbonate (PC) |
26 |
|
Polyvinyl chloride (PVC) |
29 |
|
Polysulfone (PSU) |
30 |
|
Acrylonitrile butakliene styrene (ABS) |
32 |
|
Polyether sulfone (PES) |
35 |
|
Polyamide imide (PAI) |
46 |
|
Polyether imide (PEI) |
46 |
|
Polyphenylene sulfide (PPS) |
49 |
|
Polyvinylchlorine chloride |
60 |
|
Polytetrafluoroethylene (PTFE) |
90 |
Table 1 The LOI values of some polymeric-based materials2
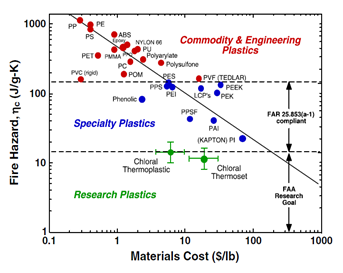
Figure 2 The plot of fire hazard (heat release capacity) versus materials’ cost. Plot courtesy: Richard E. Lyon/France, Federal Aviation Administration.3
FR applications are generally included in polymeric-based fibers in textile products applied using pad-dry-baking, spraying-baking, and steeping-drying coating methods.3–6,9,14–23,25 Thus, type of chemical used, concentration (by volume % c), viscosity (Pa.s), molecular weight (Da), pH, temperature (C), time (minutes, or hours), pressure (Pa), low free surface energy, thickness (mm), and environmental conditions (especially are relative humidity (rH), and atmospheric pressure (Po) etc.) of the coating applications were effective factors.1–30 Moreover; dip-coating, pad-dry cure, sol-gel, layer-by-layer (LbL), plasma grafting, UV curing, microencapsulation, chemical vapor storage (CVD), and co-polymerization (extrusion/mastermatch) application methods can have been also applied.3–7,10–30 As post-processes, washing (especially are the linitester, soap, alcohol, or distilled water), drying, and fixing processes were applied, respectively. FT-IR, EDS (EDX), TGA, tensile strength, SEM, thickness, grammage, vertical burning, LOI, abrasion resistance, air permeability, and washing fastness tests were applied.6,7,12,13,21–30
There are many tests carried out by scientists in FR experimental studies. The liquid oxygen index (LOI) values (thanks to ISO 4589), bond types, bond changes, bond formations, bond shifts, and bond breaks in the chemical structures of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the FT-IR test. The atom types, and percentage (%) changes in the chemical structures of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the EDS (EDX) test. The thermal degradation temperatures (Td - °C), and mass (mg) losses of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the TGA test. The technical parameters such as elasticity modulus (E), tensile strength (MPa), maximum breaking force (N), maximum percentage breaking elongation (%), elastic elongation (%), permanent elongation (%) (creep), and work at break (N.m) values of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to tensile strength (ASTM D5034 and ASTM D5035) test. The changes in the surface morphologies of polymeric-based fibers (fiber breaks, chemical distribution, particle distribution, etc.) in textile products to which FR chemicals are applied to determined thanks to SEM test. The thickness values of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the thickness (ISO 5084) test. The grammage values of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the grammage (ASTM D3776) test. After applying a flame to polymeric-based fibers in textile products to which FR chemicals have been applied for 12 seconds, the post-flame time (the time during which the material continues to burn, after the burner is removed), the final glow time (length), the time during which the material glows after the flame is extinguished), and character length (charring) behavior are applied to determined thanks to the vertical burning (ASTM D6413-08) test. The abrasion resistance values (between 5,000 and 50,000 cycles) of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the abrasion resistance (ASTM D4966 and ASTM D4970) test. The air permeability values of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the air permeability (ISO 9237) test. The washing fastness values of polymeric-based fibers in textile products to which FR chemicals are applied to determined thanks to the washing fastness (ISO 6330-2002) test.1–30 Generally, as the concentration value of the FR chemical increases, and especially if it is nanoparticle-added, the LOI value increases, but the concentration value varies depending on the type of polymeric fiber to which the FR is applied (PA, PE, PP, PU, PLA, PET, and PAN, etc.).1–30
Sustainable and eco-friendly Flame Retardant (FR) chemicals and their application processes
In the last few decades, chemicals in the form of hydrocarbon-based, and halogen-based compounds consisting of petroleum products, an exhaustible resource widely used in FR applications, have been considered by scientists to be harmless or minimally harmful to human health, as they are extremely harmful to human health (toxic products). The focus is on discovering and synthesizing more sustainable and eco-friendly chemicals that cause harm and proving their successful results through various experimental studies. This innovative process of chemical synthesis and discovery has been called "Green Chemistry". Based on this situation, the use of chemicals in the form of phosphorus-based compounds in FR applications has become widespread over time.6 Tannic acid (TA) and tannic acid's FeCl3, Fe(NO3)3, Mg(OH)2, ZnB, ZnO, ZnCl2, ZnSnO3, CuO2, Al2O3, SnO, TiO2, hydroxide, metal hydrates, melamine, phenolic, formaldehyde compounds, allyl-oxy-polyphosphazene, hypophosphorous acid, acrylic acid (AA), phosphoric acid, carboxylic acid (CA), stearic acid (SA), phytic acid (PA), chlorine, ethylene vinyl acetate (EVA), deoxyribonucleic acid (DNA), β-cyclodextrin, isosorbide, diphenolic acid, carbon allotropes, lignin, boron compounds, borax, borate, boric acid, ammonium pentaborate, polyborosiloxane, chicken egg, proteins (zein, and casein), enzymes, intumescent, minerals, amino acids, oils, alginate (ALG), gelatin (GEL), chitosan (CHI), 1,4-butanediol diglycidyl ether, triethylenetetramine compound, 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO), branched poly(ethylenimine) (BPEI), amine compounds, triazine compounds, aerogels, polydopamine (PDA), polyethylene glycol (PEG), 1-allyl-3-methylimidazolium bromide (AmimBr), 1-butyl-3-methylimidazoliumchloride (BmimCl), 1-butyl-3-methylimidazolium acetate (BmimAc), 1-ethyl-3-methylimidazolium acetate (EmimAc), hydroxymethylphosphonium salts, N-methylol phosphonopropionamide compounds, pentaerythritol, tetrakis-(hydroxymethyl) phosphonium salt (THP), silica nanoparticles, urea, epoxy, silicone, silane, siloxane, polyvinyl chloride (PVC), POCl3, ammonium phytate (APA), and ammonium polyphosphate (APP) like examples of sustainable and eco-friendly chemicals, which can be reacted in the argon gas (Ar2), nitrogen gas (N2), with ‘’Green Chemistry’’ concepts were developed by chemical engineers.1–30 Moreover; hypophosphorous acid, acrylic acid (AA), phosphoric acid, carboxylic-acid (CA), stearic acid (SA), phytic acid (PA), ethylene vinyl acetate (EVA), hydroxymethylphosphonium salts, N-methylol phosphonopropionamide compounds, tetrakis-(hydroxymethyl) phosphonium salt (THP), polyethylene glycol (PEG), urea, epoxy, siloxane, halogen, hydroxide, metal hydrates, melamine, formaldehyde, and phenolic in the form of compounds for FR chemicals have also been reported as harmful to health.6,7,12,14,15,17,29 The chemicals such as Ni(II)O, manganese borate, and ferrocene are also used as catalyst chemicals in FR applications.11
Polyester (PET) structures and their general properties
Polyester (PET) is widely used in film, bottles, packaging, electrical materials, clothing, military, industrial textile, and medical textile applications thanks to its relatively low cost, low gas permeability, high durability, high dimensional stability, easy processability, high hydrophobicity, high chemical inertness, high environmental resistance, high abrasion resistance, high modulus of elasticity, high tensile strength and high fatigue strength.10,11,13,15,18–23,25,26,28 It constitutes 90% of all fibers in the textile industry.15 The LOI value varies between 20% and 22%. Moreover, it is a synthetic-based polymeric material that can easily hydrolyze (pyrolysis), burn, and form melt drops since it is a thermoplastic fiber.10,13 The melting temperature (Tm - °C) of PET is 260 °C.11 FR properties can be provided to PET fibers with 5 different methods such as copolymerization, blending, pre-treatment, FR, and post-treatment. 3–5,10,14–30 Before applying FR application, an alkaline weakening pre-treatment process, or a plasma-grafting process is applied to PET fabrics. The purpose of applying these processes is to reduce the hydrophobicity of PET, and make the free radical functional end groups more chemically reactive. Thus, when FR application is applied, strong covalent bonds are formed between FR chemical and the free radical functional end groups of PET.12,17,20 The halogen (H), and phosphorus (P) compound chemicals are commonly applied to PET in FR applications. Phosphorus-based FR chemicals provide higher LOI values.15 Moreover, LOI values can increase from 22 to 33 depending on the type and concentration of FR chemical added in co-polyester (Co-PET) applications.15,17–21,23,25–30 Moreover; when FR chemicals are applied to PET, and PET/CO fabrics, the burning time increases and the burning rate decreases.4,5,17–30 When FR chemicals are applied to PET, and PET/CO fabrics, compared to pure PET, and PET/CO fabrics that the crystallinity ratio, and tensile strength value decrease but mass loss decreases less.20 The carbonization process depending on volume concentration after the burning test applied to the co-polyester (Co-PET) structure was presented in Figure 3.20
Figure 3 The carbonization process depending on volume concentration after the burning test applied to the co-polyester (Co-PET) structure.20
Various experimental studies about Flame Retardant (FR) finishing Process on Polyester (PET) fabrics
There are various application methods in FR applications. Both pre-treatment processes and the technical parameters of FR applications such as concentration (%), temperature (°C), time (hours), pH, liquor ratio (M/L), the mixing speed (rpm) differ from each other depending on these methods and the chemicals used. Moreover, they affect the physical, chemical, thermal, mechanical, and surface morphology changes in PET, or PET/CO blended textile structures. The general technical examination of various physical, chemical, thermal, mechanical, and surface morphology changes in textile structures, especially in the form of polyester (PET), and polyester (PET)/cotton (CO) blended fabrics of the flame retardant (FR) finishing processes from various experimental studies was presented in Table 2.21–30
|
The textile Fiber (s) |
The textile Structure |
The pre-treatment/Flame retardancy (FR) chemicals |
The pre-treatment process |
The flame retardant (FR) application process |
The post-treatment processes |
Technical Results |
Sources |
|
PET |
Woven fabric |
H2O+C2H6O+H2O2/DOPO |
T = 60 °C, t = 1.5 hours, n = 0.05 mol |
Sol-gel |
Drying, and Fixing |
Micro-cracks, and pores were observed on the woven fabric surface with 8% DOPO coating. Si and P atoms decreased from 4.7% to 1.5%. The reduction rate was 79% for the P atom, and 56% for the Si atom. The P atom was more effective at delaying ignition, and extinguishing fire. 1% DOPO was sufficient to stop melting, and improved FR activity. 8% DOPO had self-charring behavior, and made the LOI value maximum was 32. |
21 |
|
T = 60 °C, t = 3 saat, c% = 1, 3, 5, 8 (DOPO) |
T = 80 °C, t = 0.5 hours |
||||||
|
PET and Co-PET (BCY) |
Non-woven surface fabric |
H20+C2H6O /C7H10O3+C5H8O2+C8H8+C14H10O4 = MF resin |
T = 60 °C, t = 0.5 hours, n = 400 rpm, c% = 20 (5.4 g), pH 3.5 |
Pad-dry cure |
Drying |
As MF resin concentration (% c) increased, coating the thickness, and softening temperature (°C) increased, but air permeability decreased. Moreover; as the MF resin concentration increased, the burning time increased, and the burning rate decreased. |
22 |
|
T = 80 °C, t = 3 hours, n = 300 rpm, c% = 20 (5.4 g), pH = 3.5, P = 1 bar, V = 2.5 m/min |
T = 100 °C, t = 6 minutes |
||||||
|
Fixing |
|||||||
|
T = 130 °C, t = 3 minutes |
|||||||
|
PET |
Woven fabric |
H2O+PSY and PEK/ZnB |
Washing with wetting ionic chemical |
Pad-dry cure |
Drying |
As the ZnB concentration (c%) increased, the thermal degradation (Td) temperature increased from 350 °C to 450 °C (mass loss was 75%), the remaining material length was longer, and the LOI value increased. (LOI value was 31.3). The optimum ZnB concentration (c%) was 50 g/l. Only at a ZnB concentration (c%) of 200 g/l, no melting dripping behavior was observed. |
23 |
|
c% = 20 g/l, 25 g/l, 50 g/l, 100 g/l, 200 g/l, M/L = 1/20, t = none, pH = 5, 5.5, and 6 |
T = 120 °C, t = between 1.5 minutes and 2 minutes |
||||||
|
Fixing |
|||||||
|
T = 160 °C, t = between 1.5 minutes and 3 minutes |
|||||||
|
Washing |
|||||||
|
c% = 15 g/l Na2CO3 + c% = 20 g/l formaldehyde melamine resin (crosslinking chemical) |
|||||||
|
PET |
Woven fabric |
H2O+HCl+NaOH+TiO2/BPEI+APP+PA |
30 mL for BPEI solution (c%) = 0.24 g, pH 9, |
LBL |
None |
As the concentrations of BPEI, APP, and PA increased (c%), the thermal degradation (Td) temperature increased, and the mass loss increased. The remaining material length was longer, and the LOI value increased. Moreover; as BPEI, APP, and PA concentrations (c%) increased, the burning time increased, and the burning rate decreased. |
24 |
|
APP (c%) = 1.2 g, pH 5.6, NaOH (c%) = 5, pH 7, Impregnation |
T = 80 °C, t = 20 minutes |
||||||
|
T = 80 °C, t = 10 minutes |
|||||||
|
Washing with H2O |
|||||||
|
Drying |
|||||||
|
T = 70 °C, t = 10 minutes |
|||||||
|
PET |
Woven fabric |
H2O+NaOH+CH4N2O+NH4OH+PA/β-CD+APA |
None |
Pad-dry cure |
Drying |
As β-CD+APA concentrations (c%) increased, the thermal degradation (Td) temperature increased from 423 °C to 462 °C, there was mass loss, the remaining material length was longer, and the LOI value increased. (LOI value was 29.5). |
25 |
|
T = 70 °C, t = 20 minutes, M/L = 1:20, c% = 2, 4, 6, 8, pH 7, P = 0.3 Pa, V = 2.0 m/min |
T = 80 °C, t = 0.5 hours |
Melting dripping behavior was not observed in any of the FR chemicals applied on PET fabrics. No post-flame burning behavior was observed for PET-21%APA/4%CD, and increasing CD% ratios. It had self-charring behavior. Microcracks, and pores were observed on the woven fabric surface for all concentration values of β-CD+APA coatings. Moreover; as β-CD+APA concentrations (c%) increased, burning time increased, and burning rate decreased. |
|||||
|
PET |
Knitted fabric |
H2O+C2H6O+H2O2/DOPO |
T = 60 °C, t = 1.5 hours, n = 0.05 mol |
Sol-gel |
Drying, and Fixing |
As DOPO concentration (c%) increased, it did not change the thermal degradation (Td) temperature (400 °C), and mass loss. The remaining material length was longer, and the LOI value increased. (LOI value was 32). It had self-charring behavior. Microcracks, and pores were observed on the woven fabric surface for all concentration values of DOPO coatings. Moreover; as DOPO concentration (c%) increased, the burning time increased, and the burning rate decreased. The washing fastness value did not change after 5 washings. |
26 |
|
T = 60 °C, t = 3 hours, % c = 1, 3, 5, and 8 (DOPO) |
T = 80 °C, t = 0.5 hours |
||||||
|
PET, CO and PET/CO |
Woven fabric |
H2O+NaOH/casein |
T = 80 °C, t = none, n = 300 rpm, c% = 5 for casein, c% = 1 M for NaOH, pH 10, |
Pad-dry cure |
Drying, and Fixing |
As casein concentration (c%) increased, the thermal degradation (Td) temperature increased from 319 °C to 354 °C for CO, from 400 °C to 426 °C for PET, from 332 °C to 423 °C for PET/CO. LOI values increased from 18 to 24 for CO, from 21 to 26 for PET, and from 19 to 21 for PET/CO. It had self-charring behavior. Moreover; as casein concentration (c%) increased, the burning time increased, and the burning rate decreased. |
27 |
|
washing with distilled water |
T = 80 °C, t = 3 hours |
T = 80 °C, t = until it dries |
|||||
|
PET/CO (50/50) |
Woven fabric |
H2O+H3NSO3+HNO3+NaH+THF/allyl-oxy-polyphosphazene (PPZ) |
Plasma surface enhancement under Ar2 gas, T = none, t = 10 minutes |
UV curing |
Drying |
As the PPZ concentration (c%) increased, the thermal degradation (Td) temperature increased. The LOI value increased from 19 to 27 for PET/CO. Mass loss was approximately 3%. It had self-charring behavior. Fiber pyrolysis for CO, and microcracks, and pores for PET were observed on the woven fabric surface for all concentration values of PPZ coatings. |
28 |
|
l = 20 cm, washing with linitester |
T = room temperature (°C), t = until it dries, c% = 0.4 g (in 8 ml HNO3), T = 180 °C |
Moreover; as PPZ concentration (c%) increased, the burning time increased, and the burning rate decreased. No change was observed on the woven fabric from 5,000 to 50,000 cycles for abrasion resistance. The washing fastness value did not change after 6 washings. |
|||||
|
PET/CO (80/20) |
Woven fabric |
H2O+C6H5CH3+NaPO2H2 +C2H6O/PA+pentaerythritol+boric acid |
T = between 110 °C and 150 °C, t = between 0.5 hours and 2.5 hours, |
Pad-dry cure |
Drying and Fixing |
As the PA+pentaerythritol+boric acid concentration (c%) increased, the thermal degradation (Td) temperature increased from 370 °C to 435 °C for CO. Mass loss was 13.7%. The LOI value increased from 16.9 to 31.7 for PET/CO. It had self-charring behavior. Fiber pyrolysis for CO, and microcracks, and pores for PET were observed on the woven fabric surface for all concentration values of PA+pentaerythritol+boric acid coatings. Moreover; as the PA+pentaerythritol+boric acid concentration (c%) increased, the burning time increased, and the burning rate decreased. The washing fastness value did not change after 10 washings. |
29 |
|
c% = 100 g/l and 400 g/l, washing with C2H6O |
M/L = 1:25, T = 60 °C, t = 0.5 hours |
T = 160 °C, t = 3 minutes |
|||||
|
PET/CO (70/30) |
Woven fabric |
H2O+HNO3+HClO4/PVA+ H3PO4+DCA+CH4N2O |
T = between 70 °C and 100 °C, t = between 0.5 hours and 2 hours, % c = 50 g for PVA and c% = 2 g for CH₄N₂O |
Pad-dry cure |
Drying |
As PVA+ H3PO4+DCA+CH4N2O concentration (c%) increased, the thermal degradation (Td) temperature increased. Mass loss was from 10.5% to 25.9%. The LOI value increased from 17 to 36 for PET/CO. The ideal pH was 7. It had self-charring behavior. For all concentration values of PVA+ H3PO4+DCA+CH4N2O coatings, fiber pyrolysis for CO, and microcracks, and pores for PET were observed on the woven fabric surface. Moreover; as the PVA+ H3PO4+DCA+ CH4N2O concentration (c%) increased, the burning time increased, and the burning rate decreased. After 10 washings, the washing fastness value was almost gone. After 10 washes, the LOI value decreased from 36.7 to 26.4. P-N bonds increased FR activity. |
30 |
|
M/L = 1:10, T = cooled from 70 °C to room temperature (°C), t = 0.5 hours, pH = 4, 7, and 10, pH adjustment = 0.1 mol/l with H3PO4 or mol/l with NH3.H2O |
T = 90 °C, t = 5 minutes |
||||||
|
Fixing |
|||||||
|
T = 110 °C, t = 3 minutes |
Table 2 The general technical examination of various physical, chemical, thermal, mechanical, and surface morphology changes in textile structures, especially in the form of polyester (PET), and polyester (PET)/cotton (CO) blended fabrics of the flame retardant (FR) finishing processes from various experimental studies21–30
In the first main part of this theoretical review study included that the FR finishing process was examined technically from its purpose, history, applications, mechanism, effective factors, effective technical parameters, LOI values of some polymeric-based materials, used chemicals, application methods, various tests, and standards applied. In the second main part of this theoretical review study was examined technically from sustainable and eco-friendly FR chemicals, and their application processes. In the third main part of this theoretical review study was examined technically from various physical, chemical, thermal, mechanical, and surface morphology changes in PET, and PET - CO blended structures. In the last main part of this theoretical review study was made from various technical interpretations related to various experimental studies, especially from various physical, chemical, thermal, mechanical, and surface morphology changes in structures with PET, and PET - CO blended fabric forms.
The growing demand for sustainable solutions for FR in PET fibers raises questions about the efficacy, and environmental impacts of these products. The main purpose of this theoretical review study was to examine the technical aspects of various physical, chemical, thermal, mechanical, and surface morphology changes in PET, and PET - CO blended fabric structures, especially in their FR finishing processes and to guide future technical studies. FR application was applied to reduce the flammability of flammable textile products, and extend the burning time by giving FR properties to polymeric materials. FR applications were military protective clothing, firefighter clothing, workers' clothing in the metal industry, baby clothing, military textiles, sports textiles, construction textiles, geotextiles, automotive, electrical, upholstery seat fabrics, carpet, and home textile products. FR applications generally occured by adding FR chemicals as low molecular weight (Da) additives to polymeric-based-materials, and creating strong covalent bonds between the polymeric-based fiber, and FR chemicals. The type of chemical used, concentration (by volume c%), viscosity (Pa.s), molecular weight (Da), pH, temperature (C), time (minutes, or hours), pressure (Pa), low free surface energy, thickness (mm), and environmental conditions (especially are relative humidity (rH), and atmospheric pressure (Po) etc.) of the coating applications have been effective factors on FR applications. Moreover; dip-coating, pad-dry cure, sol-gel, layer-by-layer (LbL), plasma grafting, UV curing, microencapsulation, chemical vapor storage (CVD), and co-polymerization (extrusion/mastermatch) application methods can be applied. The washing (especially are the soap, alcohol, or distilled water), drying, and fixing processes, respectively, have been applied as post-processes.
FT-IR, EDS (EDX), TGA, tensile strength, SEM, thickness, grammage, vertical burning, LOI, abrasion resistance, air permeability, and washing fastness tests have been applied. LOI test has also been the most important test, too. It must be over 21% ratio. Triazine, formaldehyde, melamine, halogen (H), phosphorus (P) compound chemicals, ZnB, and silica (Si) nanoparticles have been commonly used as non-sustainable and non-eco-friendl chemicals. In conclusion, before applying FR application, alkaline, or plasma grafting pre-treatment processes should have been applied. Phosphorus (P)-based FR chemicals provide higher LOI values. (LOI value are 33).
How to explain this work advances, or refines the current understanding of sustainable FR’s, and their applications in textile materials is important for this study. Thus; polydopamine (PDA), chitosan (CHI), casein, protein, enzyme, DOPO, APA, β-CD, and boric acid have been commonly used as sustainable and eco-friendly chemicals. They are also FR effectiveness, low smoke, and toxicity, biocompatibility, and cost-efficiency chemicals. In conclusion, the alkaline, or plasma grafting as pre-treatment processes should be applied before applying FR applications. They can be applied the lower concentrations (by volume c%), temperatures (°C), times (minutes, or hours), pH’s, and higher mixing speeds (rpm) than non-eco-friendly chemicals in FR applications. Moreover, they can provide extended burning times, and shortened burning rates, higher washing fastness values, and self-charring behaviors on PET, or PET/CO blended textile structures.
The optimization for FR application should be varied between 8% and 20% (by volume c%) for the concentration, between 60 °C and 80 °C for the temperature, between 0.5 hours and 3 hours for the time, 7 for pH, 1:10 for flotte ratio with using pad-dry cure, or sol-gel processes. The between 80 °C and 160 °C for temperature for between 3 minutes and 5 minutes for time with distilled water, or ethanol chemicals, which are washing chemicals as drying, and fixing processes, respectively. As FR concentration increases (by volume c%), mass loss, and burning time increase, and the burning rate decreases. It has also self-charring behavior, too.
None.
None.
The authors declare that there is no conflict of interest.

©2024 Turşucular. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.