Journal of
eISSN: 2574-8114


Research Article Volume 4 Issue 2
Facility for Ecological and Analytical Testing, Indian Institute of Technology, India
Correspondence: Padma S Vankar, Facility for Ecological and Analytical Testing, Indian Institute of Technology, 204 A Southern Block, Kanpur-208 016, India
Received: October 22, 2017 | Published: March 1, 2018
Citation: Vankar PS, Shukla D, Spectrum of colors from reseda luteola and other natural yellow dyes. J Textile Eng Fashion Technol. 2018;4(2):105-118. DOI: 10.15406/jteft.2018.04.00127
In our search for a good natural yellow dye, Reseda luteola L fresh extract of the flower, stem and leaves was found to have good colorant quality. As the dyed cotton, silk and wool fabrics as well as yarn showed very good wash and light fastness properties, exploration of dyeing potential of this wonderful dye extract under different pretreatment conditions, mordanting- pre and post with metal mordants, biomordant, enzymes and surfactants were carried out. Our study with Reseda extract showed the following results- bright yellow color with alum, olive green with copper sulphate and dark brown with ferrous sulphate. Sodium salt of Dodecyl benzene-sulphonic acid (SDBS) and Cetyl trimethyl ammonium bromide (CTAB) as pretreatment have also been used. Combination of other natural dye extracts with Reseda extract has been attempted. A wide spectrum of colors has been obtained ranging from canary yellow to olive green to orange.
Keywords: reseda dye, mordants, biomordants, surfactants, combination of natural dyes
Yellow dyes have always been used from ancient times and have been a color of marking festivity. Most of the yellow dyes are flavonoids which are not stable to UV radiation. The dyed fabric shows very poor wash and light fastnesses. In our quest for a stable yellow dye we started exploring all the possible sources of yellow natural dyes ranging from Curcuma rhizome, Eucalyptus barck Super Critical Fluid Extract (SCFE), Gaillardia flower, Thevetia peruviana flower, Tegetus flower, Punica granatum fruit epicarp, Cassia fistula flower, Cosmos sulphuerus flower, Carthamus flower and Reseda luteola flower, stem and leaves extracts. Since a lot of work had already been done for the identification of colorant in Reseda extract, some very pertinent questions arose to understand the mode of attachment of this colorant molecule on the fabric for stable dye adherence and unusual good fastness properties. Owing to its good thermal stability, the colorant can be extracted easily with water at alkaline pH or even with methanol: water mixtures.
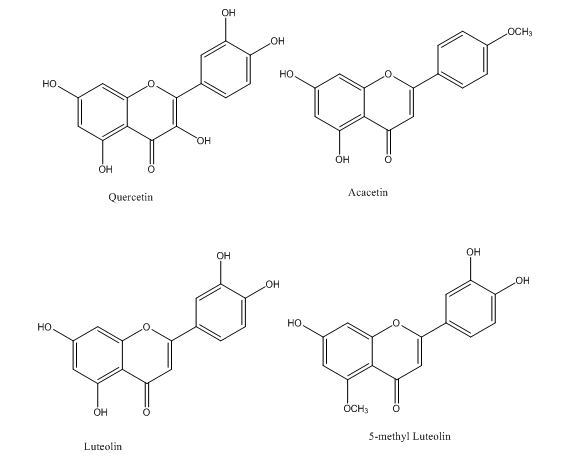
A method was applied to evaluate the influence of soil fertility on the production of flavonoids in Reseda dye. The results showed that dye capacity is dependent on soil fertility and the origin of seeds. HPLC-diode array detector (DAD) methodology was developed to allow the simultaneous identification and quantification of Reseda luteola L. (weld) dye flavonoids, luteolin, apigenin, luteolin 7-O-glucoside, apigenin 7-O-glucoside, luteolin 3',7-O-diglucoside and luteolin 4'-O-glucoside1 as shown in Figure 1. The method was developed by Gaspar et al, for the simultaneous identification of Reseda luteola L. (weld) flavonoids and quantification of the main compounds responsible for the yellow color. This method was applied to a large number of wild Portuguese welds to evaluate its potential application as dyestuff for textile factories, as a substitute for the synthetic dyes currently used. All these molecules had good chelation with metals due to presence of oxo and hydroxyl groups. The complex stoichiometry ratio of aluminium and luteolin was found to be 1:2.2
The most appropriate leaching solvent for luteolin from leaves, stems and flowers of Reseda luteola was found to be methanol optimal luteolin extraction was 8.6g/kg of plant material. Preliminary dyeing tests on pre-mordanted raw cotton and wool standard specimens gave greenish-yellow hue, acid perspiration fastness was found to be resistant to fading of dyed wool specimens was generally greater than that of cotton dyed samples.3
The structural difference in the various yellow flower extracts gives further insight into the uniqueness of Reseda extract. Curcuma rhizome has main colorant as Curcumin with some Rutin flavonoid4 as shown in Figure 2.
Gaillardia flower extract consists of Sesqiterpene lactones, aglycones and glycosilated flavonoids, dihydroxy flavonol and 6-methoxyethers5 as shown in Figure 3.
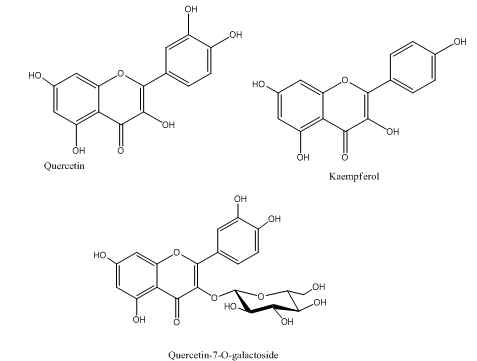
Thevetia peruviana flowers have main flavonoids as quercetin, Kaempferol and quercetin-7-O-galactoside6 as shown in Figure 4.
Cosmos sulphureus flower extract consisted of as Fustin and quercetin7,8 as shown in Figure 5.
Tegetus flower consists of Patuletin and Patulitrin flavonoids as their main constituents9 as shown in Figure 6.
Punica granatum consist of Myricetin, quercetin, Luteolin and Kaempferol in the epicarp of its fruit10 as shown in Figure 7.
Cassia fistula yellow flower has Kaempferol, Catechin and proanthocyanidins as the main flavonoids11 as shown in Figure 8.
Carthamus tinctorius is also another plant with flavonoids such as acacetin, luteolin, quercetin, and their glucuronide, cinaroside, 5-O-methylluteolin and rutin12 as shown in Figure 9.
The Supers Critical fluid extract of Eucalyptus Bark also is rich in flavonoids such as Eriodictyol, naringenin and isorhamentin13,14 as shown in Figure 10.
It well known that flavonol based molecules- quercetin and kaempferol are found to be more prone to light induced fading than flavones based luteolin and apigenin.15 Infact the latter ones darken with time.
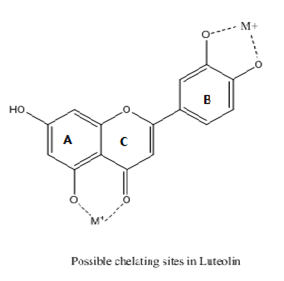
The preferred binding site depends on the flavonoids, metal and the pH. Even the binding sites differ for flavonol and flavones with Al+3 at different pH. At alkaline pH flavonols have strongest affinity with Al+3 through their ortho dihydroxy group while flavones show binding through site 4 and 5 of A and C rings or the catechol sites in ring B16,17 and shown in Figure 11.
Most of the yellow dye plants contain colorant molecule from the group of hydroxyl flavones, however the combination of components may differ from plant to plant and species to species.18 Some plausible explanation can be given to the fact that the Table 1 shows such vast difference in wash, light fastnesses, solubility and dyeability of the natural extracts (Figure 12) (Figure 13). It is very apparent that among 10 different sources, Reseda excels in all the three parameters (Tables 2) (Table 3) (Figure 14).
Dye plant |
Wash fastness |
Light fastness |
Dyeability |
Solubility in water |
Reseda |
Very Good |
Very Good |
Very Good |
Good |
Gaillardia |
Poor |
Poor |
Very Poor |
Poor |
Thevetia |
Poor |
Poor |
Poor |
Poor |
Tegetus |
Good |
Good |
Good |
Poor |
Punica |
Good |
Good |
Good |
Good |
Cassia |
Very poor |
Very Poor |
Very Poor |
Poor |
Cosmos |
Good |
Good |
Good |
Poor |
Carthamus |
Good |
Good |
Good |
Poor |
Curcuma |
Poor |
Poor |
Poor |
Good |
Eucalyptus SCFE |
Good |
Good |
Good |
Poor |
Table 1 Relative dyeability and fastness properties of extracts
CIE lab |
Reseda |
Rl + maddar |
RL+catechu |
RL+eupatorium |
RL+indigo |
L* |
64.565 |
43.242 |
15.778 |
48.442 |
64.867 |
a* |
-6.463 |
14.572 |
25.994 |
7.394 |
-33.62 |
b* |
47.577 |
42.009 |
27.175 |
45.367 |
12.703 |
C* |
48.014 |
44.465 |
37.605 |
45.966 |
35.94 |
H* |
97.769 |
70.841 |
46.254 |
80.711 |
159.31 |
dE* |
----- |
30.465 |
62.047 |
21.374 |
44.202 |
Color |
Yellow |
Orange |
Brown |
Olive green |
Turquiose |
Table 2 Reseda extract with different mordants
CIE lab |
Reseda |
Rl + maddar |
RL+ctechu |
RL+eupatorium |
RL+indigo |
L* |
64.565 |
43.242 |
15.778 |
48.442 |
64.867 |
a* |
-6.463 |
14.572 |
25.994 |
7.394 |
-33.62 |
b* |
47.577 |
42.009 |
27.175 |
45.367 |
12.703 |
C* |
48.014 |
44.465 |
37.605 |
45.966 |
35.94 |
H* |
97.769 |
70.841 |
46.254 |
80.711 |
159.31 |
dE* |
----- |
30.465 |
62.047 |
21.374 |
44.202 |
Color |
Yellow |
Orange |
Brown |
Olive green |
Turquiose |
Table 3 Reseda extract in combination with other natural dyes
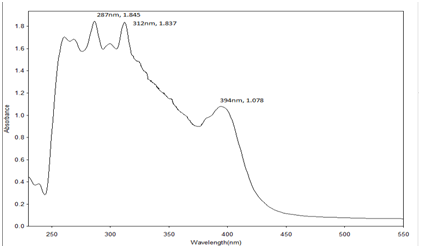
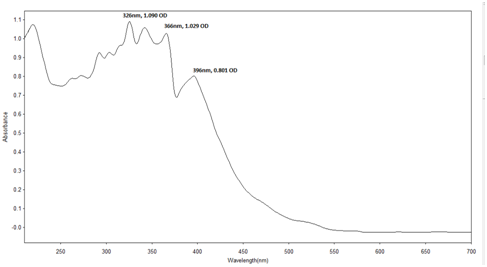
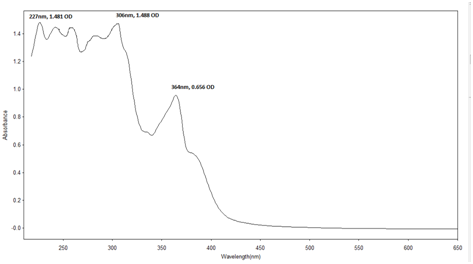
UV-Visible analysis of reseda dye and other yellow dye yielding plants: The UV- Vis spectra of most of the flavonoids show two main absorption bands such as a) the benzoyl band at 240-280 nm range and b) the cinnamoyl band at 320-385 nm range.15
Reagents: all reagents should be of analytical purity: Methanol, Reseda dye and other natural yellow extracts
Apparatus and equipment: Ultra Violet -Visible spectrophotometer machine, Quartz sample tube (cuvette) having 1.00 cm light path.
Sample preparation: The Reseda dye was weighed separately (0.1gm) in 1000 ml methanol and scanned through UV-Visible spectrometer. For visible spectrum this solution is used and for UV spectrum the solution is further diluted by 5 times. Identification of the dye by this method is through the ultraviolet (UV) region scanning from 200 to 400 nm, and the visible portion is from 400 to 800 nm (Table 4) (Figures 15−21).
Flower extract |
Benzoyl band 240-280 nm |
Cinnamoyl band 320-385 nm |
Reseda |
268 |
348 |
Curcuma |
244 |
367 |
Tegetus |
234 |
402 |
Punica |
227 |
306 |
Cosmos |
274 |
382 |
Carthamus |
||
Cassia |
247 |
398 |
Gaillardia |
272 |
388 |
Thevetia |
291 |
308 |
Eucalyptus bark SCFE |
272 |
390 |
Table 4 Identification of the dye by this method is through the ultraviolet (UV) region scanning from 200 to 400 nm, and the visible portion is from 400 to 800 nm
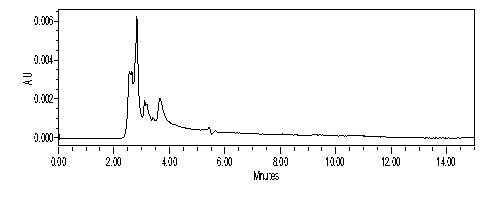
Different yellow flowers and natural reseda dye by HPLC method
Reagents: All reagents should be of analytical purity: Methanol, De-ionised water, Ethyl acetate (HPLC grade)
Apparatus and equipment: HPLC Machine (Waters), Ultra Violet-Visible Detector (Waters 2998), C-18 reverse phase column (RPC -C18), Binary pump system, Micro syringe
Sample preparation: All the different types of yellow flowers were extracted in methanol, while Reseda dye sample was weighed separately (0.1gm) in 1000 ml of methanol and then the sample is diluted to 100 times, 1μL of this diluted prepared solution was injected to the C-18 reverse phase column and eluted through column. The base line showed response within a run of 15 mins for natural Reseda dye. The parameters of this assay were made to be such that a clean peak of the both the samples are observed from the chromatograph.
Method used
The experimental conditions are as following: Column C18, 150×4.6 mm; flow rate: 1.0 ml/min; detection wavelengths 255 nm (band width 16 nm), Solvent System: Methanol: Deionised water (97: 03) in method-I and EtOAc: MeOH (10:90) in method -II has been used, Pump Pressure: 15 MPa, Machine Brand: Waters. Clear observation was made from the analysis of Reseda dye by two different methods as mentioned below:
Method-I
Commercial reseda powder: Solvent System: 97:03 MeOH: H2O, Run Time: 12 min, Sample prepared in MeOH, Chromatogram taken on 366nm (Figures 22−27).
Cosmos flower: Solvent System: 97:03 MeOH: H2O, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken on 255nm (Figures 28) (Figure 29).
Punica granatum: Solvent System: 97:03 MeOH: DW, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 255nm (Figures 30−35).
Carthamus flower: Solvent System: 95:5 MeOH: DW, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 255nm (Figure 36).
Carthamus flower: Solvent System: 95:5 MeOH: DW, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 280nm (Figure 37).
Eucalyptus bark CO2 extract: Solvent System: 95:5 MeOH: DW, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 255nm (Figure 38).
Eucalyptus bark CO2 extract: Solvent System: 95:5 MeOH: DW, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 280nm (Figure 39).
Punica: Solvent System: 95:5 MeOH: DW, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 255nm (Figure 40).
Punica: Solvent System: 95:5 MeOH: DW, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 280nm (Figure 41).
Curcuma powder: Solvent System: 95:5 MeOH: H2O, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 255nm (Figure 42).
Reseda powder: Solvent System: 97:3 MeOH: H2O, Time: 15 min, Sample prepared in MeOH, Chromatogram taken on 255nm (Figure 43).
Reseda powder: Solvent System: 97:3 MeOH: H2O, Time: 15 min, Sample prepared in MeOH, Chromatogram taken on 280nm (Figure 44).
Reseda powder: Solvent System: 90:10 MeOH: EtOAc, Time: 10 min, Sample prepared in MeOH, Chromatogram taken on 255nm (Figure 45).
Reseda powder: Solvent System: 90:10 MeOH: EtOAc, Time: 10 min, Sample prepared in MeOH, Chromatogram taken on 280nm (Figure 46).
Solvent System: 97:03MeOH: H2O, Run Time: 15 min, Sample prepared in MeOH, Chromatogram taken at 255nm (Figure 47).
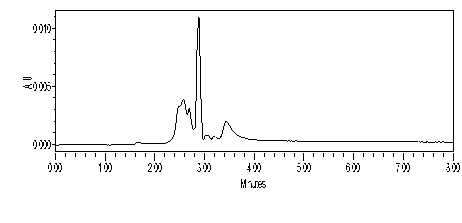
Method-II: Solvent System: 10:90 EtOAc: MeOH, Run Time: 8 min, Chromatogram taken at 255nm, Sample prepared in EtOAc (Figure 48).
Cold dyeing experiments on silk and cotton with fresh reseda extract
Dyeing method for silk and cotton: In the present study Silk and Cotton fabrics were dyed with Reseda extract from stem, flower and leaves using alum and protease enzyme for silk as pretreatment, while for cotton only alum mordant was used.
Pure Silk-The munga silk of GSM-45 fabric was scoured with solution containing 0.5 g/L sodium carbonate and 2 g/L non-ionic detergent (Labolene) solution at 40-45˚C for 30 min, keeping the material to liquor ratio at 1:50. The scoured material was thoroughly washed with tap water and air dried at room temperature. The scoured material was soaked in clean water prior to dyeing or mordanting. Cotton fabric was also scoured by standard process similar to the process mentioned herein Enzymes (Protease) was procured from TFF Speciality Chemicals, Kanpur. Alum as Metal mordant was procured from SD Fine Chemicals, Kanpur.
Dye material
The extract was prepared by using dry matter powder of stem .leaves and flowers (100g) were soaked in sufficient water 300 mL at 70-75˚C. The pH of the dyeing solution was 6.99.
Two step processes with premordanting followed by dyeing was carried out. Dyeing was carried by a stepwise dyeing process using either alum or enzyme- Protease, 1% w/w of the fabric). Similarly experiments were carried out for metal mordant--alum, and comparison of dyed swatches with enzyme treated swatches in the case of Silk fabric and only with alum 2% in the case of Cotton fabric.
The enzyme pre-treated silk fabric was used for dyeing with Reseda extract (10 %, w/w with respect to the wt. of the fabric). The dyeing time was 3 hours at a temperature of 30-40˚C.19 Dyeing was also carried out for metal mordanted silk piece (in the ratio of 2 % mordant, w/w with respect to the fabric) in a similar way keeping the dyeing time and temperature same.
Similarly premordanting with alum followed by dyeing under same conditions was carried out for cotton fabric as well.
Samples of Silk and cotton:
CIE lab |
Control silk |
Silk +alum |
Silk+protease |
Cotton control |
Cotton+alum |
L* |
73.226 |
85.545 |
71.876 |
80.487 |
90.197 |
a* |
-7.092 |
-7.578 |
-6.517 |
-5.447 |
-7.904 |
b* |
24.081 |
54.163 |
21.532 |
19.764 |
43.014 |
C* |
25.104 |
54.691 |
22.497 |
20.501 |
43.734 |
H* |
106.44 |
97.998 |
106.869 |
105.438 |
100.444 |
dE |
--- |
32.51 |
2.941 |
--- |
25.316 |
K/S |
12.796 |
102.663 |
11.341 |
6.208 |
53.968 |
Table 5 Showing the CIE Lab and K/S values for the dyed silk and cotton fabric
The dyeing results of Silk and cotton show the following: While dyeing silk fabric with Reseda dye extract, alum mordanting is superior to use of Protease enzyme in terms of dE* values and K/S values. For cotton fabric with Reseda dye extract alum mordanting shows better K/S value as well as Chroma C* and Hue color H* higher than control sample (unmordanted cotton fabric). Thus through this experiment it can be concluded that for Reseda dye extract alum mordanting is most suited for both silk and cotton fabrics and use of enzyme did not give expected results. Thus we started exploring other metal mordants with Reseda dye extract and the results are given below.
Dyeing experiments on silk and cotton with reseda samples with different metallic mordants
In the present study Silk and Cotton fabrics were dyed with Reseda extract derived from stem, leaves and flowers using alum, copper sulphate, ferrous sulphate, potassium dichromate and stannous chloride as mordant in two step dyeing process. Silk and Cotton fabrics were also scoured by standard process similar to the process mentioned earlier. Metal mordants were procured from SD Fine Chemicals, Kanpur.
Dye material
The extract was prepared by using dry matter powder of stem leaves and flowers (100g) was soaked in sufficient water 300 mL at 70-75˚C. The pH of the dyeing solution was 6.99.
Two step processes with premordanting followed by dyeing was carried out. Dyeing was carried by a stepwise dyeing process using metal mordants mentioned above in 2 %. The pre-mordanted silk fabric was used for dyeing with Reseda extract. The dyeing time was 3 hours at a temperature of 30-40˚C. Dyeing was also carried out for metal mordanted cotton in a similar way keeping the dyeing time and temperature same (Figures 50−53) (Table 6) (Table 7).
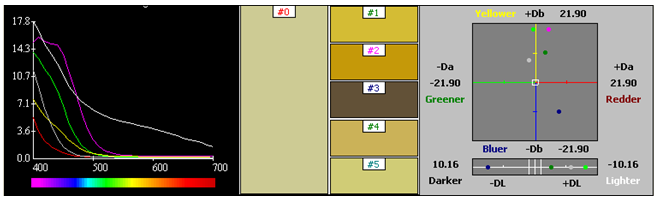
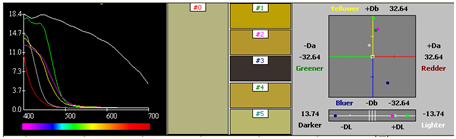
CIE lab |
Control |
KAL(SO4)2 |
CuSO4 |
FeSO4 |
K2Cr2O7 |
SnCl2 |
L* |
80.546 |
88.706 |
86.344 |
72.851 |
83.227 |
86.421 |
a* |
-7.598 |
-8.289 |
-2.85 |
0.804 |
-4.11 |
-9.836 |
b* |
27.214 |
47.114 |
47.026 |
16.243 |
38.423 |
35.451 |
C* |
28.255 |
47.838 |
47.112 |
16.263 |
38.642 |
36.79 |
H* |
105.629 |
100.01 |
93.503 |
87.131 |
96.139 |
105.537 |
dE |
-- |
21.519 |
21.182 |
15.817 |
12.041 |
10.362 |
K/S |
8.564 |
51.288 |
92.946 |
124.162 |
26.535 |
19.267 |
Table 6 Cotton - Metal mordanting
CIE lab |
Control |
KAL(SO4)2 |
CuSO4 |
FeSO4 |
K2Cr2O7 |
SnCl2 |
L* |
72.148 |
83.523 |
78.589 |
60.407 |
79.303 |
79.547 |
a* |
-8.014 |
-7.114 |
-3.629 |
3.577 |
-5.525 |
-10.586 |
b* |
26.161 |
56.8 |
48.369 |
5.036 |
47.214 |
35.318 |
C* |
27.361 |
57.244 |
48.505 |
6.177 |
47.536 |
36.87 |
H* |
107.061 |
97.172 |
94.325 |
54.592 |
96.708 |
106.715 |
dE |
-- |
32.695 |
23.535 |
26.804 |
22.374 |
12.05 |
K/S |
16.516 |
100.694 |
68.27 |
268.484 |
62.21 |
31.919 |
Table 7 Silk- Metal mordanting
The dyeing results of Silk and cotton show the following: Dyeing silk and cotton fabrics with Reseda dye extract different mordanting shows highest K/S for ferrous sulphate mordanted fabrics. For cotton fabric with Reseda dye extract the order of effective dyeing in terms of K/S values is Ferrous sulphate > >Copper> Alum> Potassium dichromate> Stannous chloride. With silk fabric with Reseda dye extract the order of effective dyeing in terms of K/S values is Ferrous sulphate > > Alum> Copper> Potassium dichromate> Stannous chloride. We did more experiments with different surfactants and Reseda dye.
Dyeing experiments on silk and cotton with reseda samples with alum metallic mordant and surfactant
In the present study Silk and Cotton fabrics were dyed with Reseda extract using alum, as well as surfactants such as Sodium salt of Dodecylbenzenesulphonic acid (SDBS) and Cetyl trimethyl ammonium bromide (CTAB) as pretreatment have been used in two step dyeing process.
Silk and Cotton along with knitted variety D100% fabrics were also scoured by standard process similar to the process mentioned earlier. Metal mordant and Surfactants were procured from SD Fine Chemicals, Kanpur.
Pretreatment with surfactant
Aqueous solution of the surfactant (0.02 gm/ml) was prepared in 1:30 M:L. Silk fabric was dipped in 25 ml of surfactant solution and heated on water bath at 60-70˚C for 30-45 mins. Cotton was heated for 40 mins at 80-90˚C. Then the fabrics were left for aerial oxidation.
Dyeing
After this Silk and Cotton were dipped in 25 ml of 10 % solution of Reseda dye extract using 0.5 % NaOH separately at 1:30 MLR. Two step processes with premordanting followed by dyeing was carried out. Dyeing was carried by a stepwise dyeing process using metal mordants as well as surfactants SDBS and CTAB mentioned above. The pre-mordanted silk fabric was used for dyeing with Reseda extract. The dyeing time was 3 hours at a temperature of 40°C. Dyeing was also carried out for metal mordanted cotton and surfactant pre treated in a similar way keeping the dyeing time and temperature same. .Dyed fabrics were washed with water, followed by mild soap and finally with water (Figure 54) (Figure 55) (Table 8) (Table 9).
CIE lab |
Control |
KAL(SO4)2 |
SDBS |
CTAB |
L* |
73.305 |
84.219 |
71.99 |
81.373 |
a* |
-8.397 |
-7.482 |
-7.039 |
-8.363 |
b* |
30.219 |
56.691 |
27.278 |
46.341 |
C* |
31.364 |
57.183 |
28.172 |
47.09 |
H* |
105.559 |
97.552 |
104.55 |
100.262 |
dE |
-- |
28.648 |
3.496 |
18.028 |
K/S |
15.9552 |
93.24 |
14.84 |
59.032 |
Table 8 Silk and alum, SDBS and CTAB surfactants
CIE lab |
Control cotton |
KAL(SO4)2 |
Knitted cotton alum |
SDBS |
CTAB |
L* |
78.323 |
83.267 |
87.066 |
75.761 |
81.118 |
a* |
-7.418 |
-7.493 |
-6.786 |
-7.23 |
-6.723 |
b* |
45.826 |
55.103 |
62.934 |
37.493 |
46.547 |
C* |
46.423 |
55.61 |
63.299 |
38.184 |
47.03 |
H* |
99.227 |
97.777 |
96.188 |
100.947 |
98.252 |
dE |
-- |
10.512 |
19.223 |
8.72 |
2.969 |
K/S |
18.712 |
42.248 |
98.436 |
12.615 |
38.63 |
Table 9 Cotton and knitted and alum, SDBS and CTAB surfactants
The dyeing results of Silk and cotton show the following: Cotton fabrics with Reseda dye extract along with use of surfactant did not show any improvement, infact alum mordanting still remained the best, and Knitted fabric showed better result that ordinary cotton fabric.
Dyeing experiments on silk and cotton with reseda and rubia dyes samples with alum metallic mordant
In the present study Silk and Cotton fabrics were dyed with Reseda + Rubia dyes (0.35 gm+ 0.65 gm respectively) extract using alum, as pretreatment in two step dyeing process.
Silk and Cotton fabrics were also scoured by standard process similar to the process mentioned earlier. Metal mordant (Alum) was procured from SD Fine Chemicals, Kanpur.
Dye material
The extract was prepared by using powder 1.0g was soaked in sufficient water 150 mL at 70-75˚C. Mass to liquor ratio: 1.0 g in150 mL at 70˚C for 1.5 hrs.
Two step processes with premordanting followed by dyeing was carried out. Dyeing was carried by a stepwise dyeing process using metal mordant alum in 2 %. The pre-mordanted silk fabric was used for dyeing with Reseda + Rubia dyes extract. The dyeing time was 3 hours at a temperature of 50˚C. Dyeing was also carried out for metal mordanted cotton pre treated with alum in a similar way keeping the dyeing time and temperature same (Figure 56) (Figure 57) (Table 10) (Table 11).
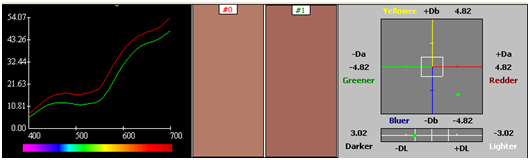
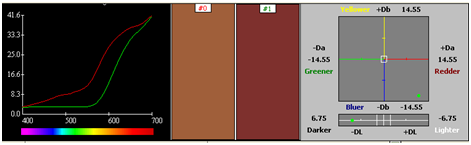
|
Standard I |
Batch 1 |
L* |
56.86 |
55.841 |
a* |
20.381 |
22.888 |
b* |
19.29 |
16.474 |
c* |
28.062 |
28.2 |
H* |
43.407 |
35.731 |
dE* |
---- |
3.906 |
DL* |
---- |
-1.019 |
Da* |
---- |
2.507 |
Db* |
---- |
-2.816 |
DC* |
---- |
0.138 |
DH* |
---- |
-3.768 |
KIS |
29.2786 |
43.568 |
RFL |
10.705 |
8.508 |
% |
100 |
148.805 |
Table 10 Cotton alum reseda+ rubia dyes
|
Standard |
Batch 1 |
L* |
46.994 |
42.242 |
a* |
23.245 |
34.59 |
b* |
31.817 |
19.271 |
c* |
39.404 |
39.596 |
H* |
53.827 |
29.112 |
dE* |
---- |
17.57 |
DL* |
---- |
-4.752 |
Da* |
---- |
11.345 |
Db* |
---- |
-12.546 |
DC* |
---- |
0.192 |
DH* |
---- |
-16.914 |
KIS |
83.1575 |
199.9764 |
RFL |
3.958 |
2.936 |
% |
100 |
240.479 |
Table 11 Silk alum reseda+ rubia dyes
The dyeing results of Silk and cotton show the following:
Among many yellow colored natural dyes Reseda seems to have the most pronounced dyeing ability and is also very good when combined with other dyes. Most of the yellow dye plants contain colorant molecule from the group of hydroxyl flavones, however the combination of components may differ from plant to plant and species to species. It shows vast difference in wash, light fastnesses, solubility and dyeability of the natural extracts. It is very apparent that among 10 different sources, Reseda excels in all the three parameters.
While analyzing Reseda by HPLC, it showed better separation by method-II, however the resolution needs to be further worked out, method –I is not so good. Better separation of peaks can be seen herein.
Dyeing Silk fabric with Reseda dye extract the order of effective dyeing in terms of K/S values Alum> CTAB> SDBS, in fact silk fabric showed the order of effective dyeing in terms of K/S values SDBS was not found to be good as pretreatment chemical for Reseda. Reseda extract showed bright yellow color with alum, olive green with copper sulphate and dark brown with ferrous sulphate.
None.
Authors declare there is no conflict of interest in publishing the article.

©2018 Vankar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.