Journal of
eISSN: 2574-8114


Research Article Volume 3 Issue 1
1AV Topchiev Institute of Petrochemical Synthesis, Russian Academy of Science, Russia
2Chemical Department, MV Lomonosov Moscow State University, Russia
Correspondence: Berkovich AK, Chemical Department, MV Lomonosov Moscow State University, Russia
Received: April 03, 2017 | Published: October 16, 2017
Citation: Kulichikhin VG, Golova LK, Makarov IS, et al. Hybrid cellulose-pan fibers spun from mutual solutions in n-ethylmorpholine-n-oxide. J Textile Eng Fashion Technol. 2017;3(1):593-596. DOI: 10.15406/jteft.2017.03.00090
By means of high-temperature dissolution of cellulose and polyacrylonitrile copolymers (PAN) in N-methylmorpholine-N-oxide (NMMO) the mixed solutions were obtained and hybrid fibers were spun. The main attention is devoted to morphology of mutual solutions (double emulsions), which changed drastically depending on composition and shear action. To hybrid fiber the spinning process was developed allowing us to prepare hybrid fibers combining properties of cellulose and PAN. And to tThe first stages of their heat treatment of hybrid fibers as precursors of carbon fibers were studied. The interrelationships between DSC and TG traces for neat and hybrid fibers in the temperature range up to 1000˚C are considered, and conclusion about mutual influence of each polymer on chemical transformations in hybrid fibers was done.
N-methylmorpholine-N-oxide (NMMO) is well known cellulose solvent and commodity cellulose fibers are produced from solutions in this solvent under trade name Lyocell.1 The melting point of anhydrous NMMO is around 200 °C that is extremely high for any polymers including cellulose. But this solvent easily forms crystal-hydrates of various compositions, and its dissolution capability related to cellulose starts from NMMO monohydrate with melting point of ~85 °C. Anyway, at ambient temperature NMMO with minor water content is solid. Basing on this feature of NMMO, the new method of dissolution via so-called solid-phase activation was developed in our group, consisting in mechanochemical treatment of cellulose and solvent powders leading to formation of solid solutions. The main mechanism of such a treatment is formation of H-complexes between solvent and cellulose under high pressure and intensive shear.2,3 At heating to temperature higher than melting point of NMMO the solid solution transforms to the viscoelastic liquid, i.e. to the dope for fiber spinning. This approach allowed us to obtain dopes with cellulose content higher than 15% and to spin cellulose fibers under trade name Orcell.2 The technical yarns of this kind were used as precursors for preparing carbon fibers of reasonable quality with tenacity higher than 1 GPa.
The other precursors of carbon fibers are copolymers based on acrylonitrile (PAN), containing such co-monomers as methyl acrylate and acidic component. As a rule, a content of co-monomers does did not exceed 10%. As a rule, PAN copolymers do not dissolve in MMO and only applying Using the above mentioned solid-phase activation procedure, we could dissolved the first time in NMMO several PAN copolymers, containing acidic groups, and prepared solutions with concentration up to 40% were prepared.4
The main aim of this research was too consisted in preparation of mixed solutions of cellulose and-PAN in the same solvent of N-methylmorpholine-N-oxide (NMMO) for, spinning their hybrid fiber spinning and to undertake the analysis of the mixed solution of dissolved cellulose-PAN at the first stages of their spinning process carbonization.
As PAN samples, we used traditional copolymers of acrylonitrile, methylacrylate and either itaconic or acrylic acid with molecular weights of (80-100)×103 synthesized in supercritical CO2 medium.5 NMMO was supplied by Shanghai Demo Medical Tech. Co, China. Sulfate cellulose in powder-like form was produced by Baikal Cellulose-Paper Mill, Russia.
The morphological peculiarities of the PAN/cellulose-NMMO systems were studied using polarizing microscopy (microscope, VEB Kombinat Nadema GmbH, former DDR). Fiber spinning was performed in laboratory home-made stand of dry-wet method. The mixed solution was extruded under heat and pressure into an air gap before it entered a water bath. The produced fibre was then washed and dried before it was heat treated (a spinneret with a hot solution was separated from the mirror of coagulant bath with an air gap on a distance of 10-20 cm).4
Mechanical measurements for tensile strength, elastic modulus and elongation at break were carried out on Instron 1122 testing machine (England) in extension mode on a specimen basis of 10 mm with a speed of movable traverse of 10 mm/s measuring tensile strength, elastic modulus and elongation at break.
The obtained fibers were thermally heated treated up to 450 °C in an air atmosphere (stage of thermal oxidative stabilization for PAN), and DSC and TG traces were registered in apparatus for synchronic thermal analysis (STA 449 С Jupiter, Netzsch Group, Germany) in argon atmosphere with a heating rate of 10 K/min.
As was mentioned before, the mixed solutions have been prepared via solid-phase activation method with a definite way order of components introducing loading into reaction zone. By this way, tThe dopes containing 18-25% of both polymers with their each percentage mass ratio of 0.1-0.310-30% and 0.7-0.970-90% were obtained (compositions with each polymer content in a domain of 0.3-0.30-70%7 could not be used for fiber spinning spun because of gel-like behavior). Analysis of solutions morphology has shown that at small PAN content an emulsion was formed in spite of good solubility of both polymers in the same solvent (Figure 1). Moreover, the detail analysis has shown that this is so-called double emulsion: drops of PAN solution phase, distributed in cellulose solution medium, contain micro-drops of cellulose solution. At shear of cover glass of thin layer of emulsion under microscope the fibrillation of micro-drops proceeds (indicated by arrow).
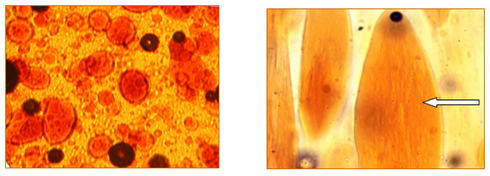
Figure 1 Morphology of the mixed 18% solution where polymer phase consists of 85% cellulose and 15% PAN at magnification ×6 (left) and ×40 (right). T=120˚C.
At prevailed content of PAN a solution is also non-homogeneous, but consists of co-existing macrophases of two solutions (Figure 2). But even in such heterogeneous system the cellulose solution phase is capable to being fibrillized even at rest, and the most prominent at shear. In other words, at flow the domains of the cellulose phase elongate in direction of shear or extension and form fibrils. According to these data, we can expect that cellulose phase in hybrid fibers will play a role of arming phase, i.e. reinforcing the PAN phase.
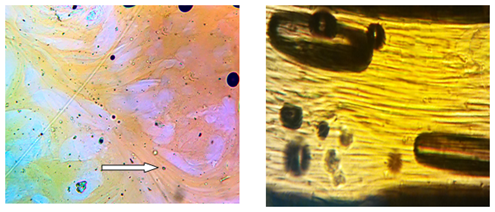
Figure 2 Morphology of the mixed 25% solution where polymer phase consists of 80% PAN and 20% cellulose at the magnifications ×40. The arrow indicates a fibrillation of cellulose phase at rest (left) and at shear (right). T=120 °C.
Hybrid fibers were spun from mixed solutions by dry-wet method (the spinneret was separated from the surface of liquid coagulant) with a dope temperature higher than 100 °C into cold water (Figure 3). A spinning velocity was around 100m/min and a spun bond hood was positive. The hybrid fibers demonstrate heterogeneous morphology. After removing the PAN phase with selective solvent (DMFA), such cellulose fibrils become visible (Figure 4).
Coupling together two absolutely different polymers: hydrophilic, cotton-like cellulose and hydrophobic, wool-like PAN led to appearance of the novel fibers which could be useful either in textile industry or in some special applications, e.g. as precursors of carbon fibers. Even at small additive of PAN (~1%) the mechanical properties of hybrid fibers change drastically: elongation at break increases in 2-3 times compared with the neat cellulose fibers.
To understand a role of PAN at pyrolysis of cellulose fibers the experiments were carried out on thermal treatment of hybrid fibers in argon atmosphere. These data are presented in Figure 5. As is seen, PAN fibers demonstrated exothermic peak at 302.5 °C that is typical for this polymer due to primary chemical transformation of macromolecules: cyclization of nitrile groups and formation of conjugated sequences along chains. But the exo-effect practically at the same temperature for cellulose fibers is not completely understandable. The matter is that for cellulose powder the endo-effect takes place at 350 °C (Figure 6) and it is interpreted as dehydrating and destruction of polymer chains. A confirmation of this explanation serves a sharp loss of mass, and derivative of the integral mass loss curve has the minimum practically repeating a shape of DSC trace (the dotted line on Figure 6). At 450 °C the mass loss for powder reaches 80%. The same time for cellulose fibers at this temperature the mass loss does not exceed 70% and remains almost constant until 1500 °C (Figure 7).
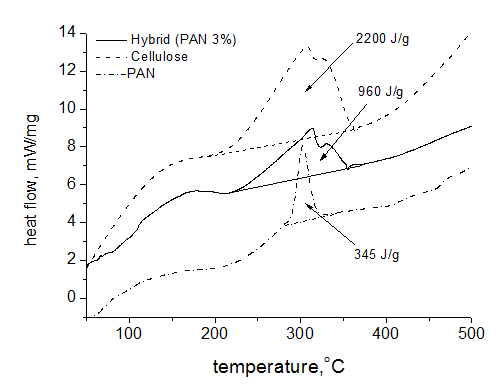
Figure 5 DSC data for hybrid fibers containing 3% PAN (solid line) and control cellulose (dash line) and PAN (dash-dot line) fibers in inert atmosphere. Heating rate 10 K/min, argon stream 50 ml/min, and crucible.
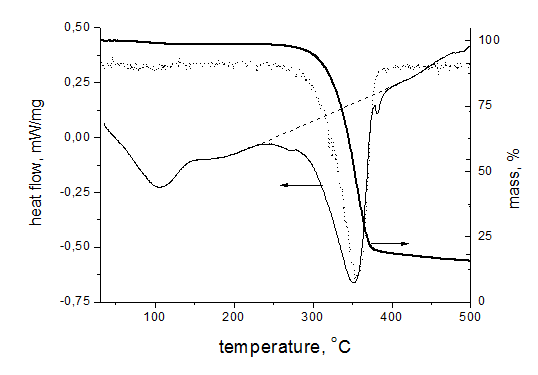
Figure 6 DSC and TG data for cellulose powder in inert atmosphere. The dotted line shows the derivative of the integral mass loss curve. Heating rate 10 K/min, argon stream 50 ml/min, Al crucible.
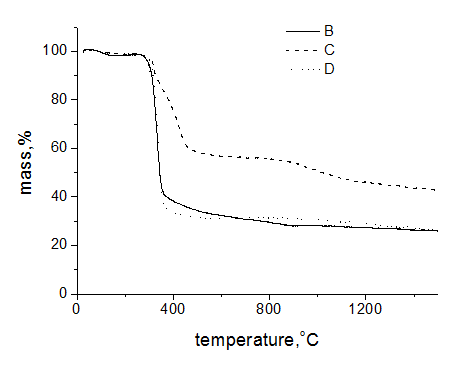
Figure 7 TG data for hybrid fibers containing 3% PAN (solid line), control cellulose (dotted line) and PAN (dash line) fibers in inert atmosphere. Heating rate 10 K/min, argon stream 50 ml/min, Al2O3 crucible.
Contradictions in thermal behavior of powder and fiber mean that at transition to oriented fibers the thermal stability of cellulose macromolecules increases sufficiently and heat flow appears as a result of structure change in macromolecular ensembles. Coming back to DSC traces it is evident that exo-peak for cellulose fibers has shoulder at 325.4 °C and its existence can be explained by superposition of exo- and endo-effects. A similar shape of this peak for the hybrid fibers says about the same origin in both cases, namely superposition of two processes with the opposite direction: destruction and structuring. The most confused question concerns the mechanism of possible structure formation causing the positive heat flow.
There are some opinions on this issue. One of them is based on rearrangement of the H-bonds network in cellulose at high temperature and release the macromolecular mobility. Since cellulose is semi-crystalline polymer (a fraction of crystalline phase is 30-40%), destruction of H-bonds, connected macromolecules in crystallites, can lead to collapse of 3D structure and formation of 2D or only orientational order, similar to liquid crystalline phase. Of course, this process should proceed with heat absorption. However, if amorphous phase of cellulose will accompany such kind of structure transformation, the fraction of partially ordering phase becomes almost 100% and the total heat evolution due to formation of intermediate mesophase can exceed heat absorption due to transition of crystalline phase to liquid crystalline state.
The other reason concerns a possible ordering of the destruction products. One of them is easily crystallized levoglucosan derivatives.6 Its fraction in the pyrolysis products increases, if the neat cellulose fibers have well ordered crystalline structure. Cotton and Lyocell (Orcell) form much more levoglucosans, than rayon. Presumably, namely crystallization of these by-products gives rise to heat evaluation.
From above mentioned viewpoints a role of PAN in cellulose can be considered as following: either it prevents the structure reconstruction of cellulose or influences on levoglucosans yield. Anyway, a decrease of heat evaluation on the first stage of pyrolysis makes carbonization process more smooth that prevents the local overheating.
As the main result of this paper the new solvent for PAN was found-NMMO that allowed us to prepare the mixed solutions of two absolutely different polymers: cotton-like cellulose and wool-like PAN. From morphological view-point mixed solutions are ordinary or double emulsions with prominent effect of the cellulose phase fibrillation. The hybrid fibers were spun by dry-wet method. Removing the PAN from extrudates/fibers with the selective solvent (DMFA) confirmed the arming effect of cellulose phase. DSC and TG traces have shown appearance of exoeffect for cellulose powder and endoeffect in approximately the same temperature range for cellulose fibers. Some hypothetic versions for explanation of this contradict behavior are considered.
The work is supported by the Russian Federation President’s Grant for young scientists MK-6543.2016.3 and Russian Science Foundation (Agreement 17-79-30108).
Author declares there is no conflict of interest in publishing the article.

©2017 Kulichikhin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.