Journal of
eISSN: 2574-8114


Research Article Volume 4 Issue 4
Department of Textile Engineering, Uludag University, Turkey
Correspondence: Tuba Toprak, Textile Engineering Department, Uludag University, Gorukle Campus, Bursa, Turkey, Tel 905070099345
Received: June 06, 2018 | Published: July 11, 2018
Citation: Toprak T, Anis P. Enzymatic decolorization of reactive dyeing baths. J Textile Eng Fashion Technol. 2018;4(4):275-278. DOI: 10.15406/jteft.2018.04.00156
Increasing social awareness and social cognition about environment are challenging the textile industry, which has highly coloured waste water. For this reason, in this study, enzymatic decolorizations of three different coloured reactive dyeing baths containing soda, salt and reactive dyes with laccase were studied. The maximum absorbance of the red coloured bath showed hypsochromic shift after enzymatic decolorization, i.e. shifted towards the blue region. The percentage of colour removal was the highest in the red and the lowest in the orange due to their tinctorial strengths. The highest colour removal among three colours were observed at 0.5 and 2% dyestuff concentrations, which indicated that laccase could be used successfully in decolorization of textile waste water.
Keywords: enzymatic decolorization, environmentally friendly, sustainability, laccase, reactive dyeing, absorbance, tinctorial, hypsochromic
The global climate change, increasing environmental pollution due to growing world population, and fast-growing industrialization resulted in rapid consumption of resources and increase in amount and variety of waste. All these developments are worrying and have forced industries and scientists to take measures against these adversities. Destruction of the natural environment we live in, which is not going to return, has been ongoing from the very old days, and recent attempts to reduce environmental pollution are very recent. Since 1980, movements towards conservation of natural life and environment have come into prominence and consumers in many countries have started to prefer products made with materials and methods that do not harm the environment during production, and post-use disposal phases.1 The widespread environmental impact of the textile industry is manifested by discharge of high amounts of chemicals to the environment.2 The various dyestuffs used in the textile industry are being discharged in large quantities during the production process.3 This is the beginning of a process that is difficult to compensate for environment and human health.4,5
Biological approaches evaluated to reduce negative effects of waste water containing dyestuffs and chemicals have gained importance. Suggested chemical and physical treatment processes have some disadvantages such as high cost, formation of toxic by-products, excessive consumption of energy, formation of concentrated sludge, and non-adaptability to all waste water in different characters.6–9 Therefore, biological approaches have been becoming more advantageous. The laccase enzyme, a member of peroxidases used for remediation of textile dyes, is the most promising enzyme since it can be operated without expensive cofactors.10–12 Laccases are also increasingly used in many industrial scales such as delignification, biological remediation agents, ethanol production, biosensors, and bio fuels as well as dye removal.13–22
Cotton, the most widely used natural fiber in the world, needs high amounts of water during its growing and processing. The most commonly used dyestuff for dyeing this fiber is reactive dyestuff, which is used and produced 80 000tons per year. Considering that about 70-150litres of water, 0.6-0.8kg of NaCl and 30-60g of dyestuffs are consumed for one kilogram of cotton dyeing,23 the amount of pollution in the waste water that is released after dyeing is frightening. Salt, coloured and organic matters load of the wastewater discharged especially in the washing processes which are repeated in many baths after the dyeing is very high. The waste water is coloured because 20-30% of the dyestuff used in dyeing is hydrolysed.24–26
The reactive dyeing via exhaustion method is faced with increasingly aggressive environmental protection measures. In this regard, with this paper decolorization of wastewater after reactive dyeing of cotton fabrics was operated with commercial laccase enzyme. It was tried to determine the amount of colour removal after enzymatic decolorization of three different colour reactive dyes at three different concentrations used in the dyeing of cotton fabric.
In the dyeing process, Setazol Red / Blue / Orange PLF reactive dyes were used as three different concentrations (0.5, 2, 4%) together with salt (NaCl, 60g/l) and soda (Na2CO3, 20g/l). Dyeing processes were carried out at 80 °C for 60 minutes at pH=11 using ATAÇ Sample Dyeing Machine. The coloured wastewater after the dyeing process was treated with laccase enzyme (Setenzim Eco-L/SetaşKimya) at 50˚C for 40minutes.
The L*, a*, b*, C*, and h0 values were calculated by reflectance measurements (under D65 illuminant and 10˚ standard observer) the Konica Minolta CM-3600D spectrophotometer by Color Mission software (v.3.4.1 by Argetek). The K/S values were calculated using the Kubelka-Munk equation. The color strength (K/S) formula is presented in Equation 1.
(1)
In the Formula 1, R is the decimal fraction of the reflectance of fabric, K is the absorption coefficient, and S is the scattering coefficient.
Tinctorial strengths were calculated by using maximum absorption values of each colour. In addition to tinctorial strength, absorbance values of the dyeing and enzymatic-treated baths were determined via UV-VIS spectrophotometer.
The percentage decolorization was calculated as follows:
(2)
The amount of dyestuff remaining in the solution was calculated via Beer-Lambert law (3):
(3)
In the Formula 3, ε is the molar extinction coefficient, c is the concentration of the absorbing solution, l is the path length, and D is the absorbance.
Colour strength (K/S) values of dyed fabrics
Colour strength values of reactive dyed fabrics are presented in Figure 1. For all colours, K/S values increased due to dyestuff concentrations were increased, as expected. Orange coloured fabrics gave higher K/S at all dyestuff concentrations than the others. Fabrics dyed with blue colour had higher K/S than the red ones. The relationship between K/S and colours was also confirmed by the tinctorial strengths of dyes as shown in Figure 2.
Absorbance values of dyeing baths
The Figure 3 shows UV–VIS absorbance values of dyeing and subsequent enzymatic decolorization treatment for red colour at 500-540nm. According to Figure 3, in the visible range, the dyeing baths were clearly darker and deeper than the latter enzymatic process solutions. For red colour, maximum absorbance values were taken at 400 nm, so it shifted to blue region after decolorization and they are shown at Table 1. A shift in maximum wavelength towards longer ones revealed degradation of this dye into different transformation products.
Max. absorbance |
Characteristic absorbance |
|
Solutions |
400nm |
525nm |
Enz- 0,5% Red |
0,16 |
0,03 |
Enz- 2% Red |
0,75 |
0,08 |
Enz- 4% Red |
2,21 |
0,12 |
Table 1 Absorbance values of red colour at shifted and characteristic wavelengths after decolorization
Absorbance values of orange and blue colours at all dyestuff concentrations after dyeing and enzymatic decolorization are shown in Figure 4. UV–VIS absorbance values were observed at 600-620nm for blue, 400-440nm for orange. Approximately same absorbance values for orange and blue colours after enzymatic decolorization at 0.5% dyestuff concentrations were taken, but the difference at 2-4% concentrations increased and orange gave higher absorbance values. Red colour gave approximately same absorbance values at 0.5 and 2% dyestuff concentrations with blue colour, while the value at 4% concentration was higher than the blue, even though its tinctorial strength was the lowest. This could be explained as the amount of dyestuff bound to fiber was less, and therefore dyestuff remaining in the solution was greater amount.
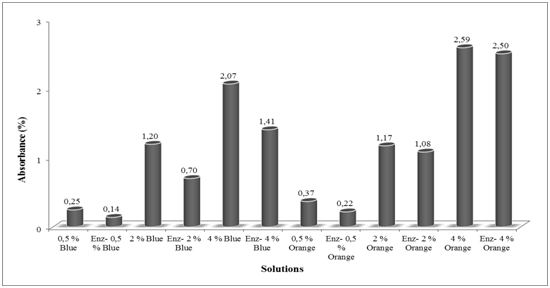
Amounts of dyestuff which is taken up by fibers and thus remained amounts in the bath after dyeing and enzymatic treatment are shown in Figure 5. After dyeing, remained colour in dye baths at the most amount was red and the least was orange for all dyestuff concentrations. The more dyestuff bound to fiber, the lesser amount of dyestuff remained in the solution. After enzymatic decolorization process, the colour remained at the maximum amount was red and thus decolorized the most as percentage which are indicated in Figure 6. This was due to the lowest tinctorial strength of this colour. Although orange and blue colours remained approximately same amount in enzymatic decolorization baths, less colour change was observed for orange, which could be interpreted by means of dyestuff structure unaffected by the enzyme and its tinctorial force, also.
Percentages of absorbance reductions are shown in Figure 6. These values showed that red colour was the most affected by the enzyme and absorbance values decreased about 56-61 % at 2% and 0.5% dyestuff concentrations, respectively. For blue colour, these reductions were about 41-43 % for same concentrations. For 0.5 % dye concentration of orange, the effect of the enzyme on decolorization was about 40 %, while the enzyme had less colour removal effect as dyestuff concentration was increased because of its tinctorial strength. As the concentration of dyestuffs used in dyeing was increased, amount of decolorized dyestuff decreased. Therefore, less decolorization percentage was observed at higher dyestuff concentrations.
UV-VIS measurements of solutions were made after dyeing and enzymatic decolorization processes with laccase. It had shown that enzymatic decolorization could be used successfully in medium and light shades, especially for red and blue colours. Red colour absorbance values were shifted in yellow, so its structure was the most affected by the enzyme. In other words, it could be said that the enzyme changed the chromophore structure of red colour. Tinctorial strengths of dyes showed inverse relationship with decolorization, so orange was the least colourless. In order to be able to explain the reaction between dyestuff and enzyme, it is necessary to analyse structure of dyestuff and to make more detailed analyses.
In summary, laccase can help colour removal of waste water, which is one of the environmental pollution crimes of the textile industry. Moreover, it has a very important advantage that there is no investment cost.
None.
Author declares there is no conflict of interest in publishing the article.

©2018 Toprak, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.