Journal of
eISSN: 2373-6410


Case Report Volume 4 Issue 2
1National Institute of Clinical Neurosciences, 57 Amerikai út, Hungary
2Semmelweis University School of PhD Studies, János Szentágothai Doctoral School of Neurosciences, Hungary
3Department of Neurology, Semmelweis University, Hungary
Correspondence: András Horváth MD, National Institute of Clinical Neurosciences, 57 Amerikai út, 1145-Budapest, Hungary, Tel 36305421019
Received: December 10, 2015 | Published: January 29, 2016
Citation: Horváth A, Szucs A, Barcs G, Kamondi A (2016) The Value of Long-Term EEG in the Diagnosis of Epilepsy in Alzheimer’s Disease. J Neurol Stroke 4(2): 00125. DOI: 10.15406/jnsk.2016.04.00125
Evidence suggests that Alzheimer’s disease (AD), the most common neuro-cognitive disorder has a strong relation to epileptic seizures both in animal models and in human Alzheimer’s patients. However, the significance and precise mechanism of this mutual tie has not yet been clarified.
Epilepsy may aggravate the cognitive decline, but non-convulsive seizures are especially hard to identify, and might even be misdiagnosed for primary dementia.
In our study, we demonstrate the occurrence and various clinical presentations of temporal lobe epilepsy in AD patients, and show the importance of long-term EEG monitoring in detecting epilepsy in dementia.
Since epilepsy has serious negative impact on cognitive performance and disease progression in AD, the early detection of epileptic events is crucial. We suggest that in the dementia patients prolonged EEG/video-EEG recording lasting at least 24 hours and containing slow wave sleep should be performed to facilitate the detection of ictal and interictal events. It is important to enhance the diagnostic accuracy of seizures in AD for identifying epilepsy as a treatment target and initiating appropriate therapy.
Keywords: Alzheimer’s disease, Epilepsy, Seizures, EEG, Non-convulsive status epilepticus, Long-term EEG
Major neuro-cognitive disorders (NCD) constitute an increasing problem of our aging society. Alzheimer’s disease (AD) is the most common cause of NCD, making ponderous medical and economical burden.1 Its main symptoms are cognitive decline with loss of memory, impairment of behavior and language skills. Typically, the cognitive performance of Alzheimer’s patients shows significant fluctuations2 that may resemble to seizure-related behavioral changes seen in non-convulsive epileptic seizures.3
The risk of epileptic seizures is higher in conditions with NCD than in non-demented controls.4 There is growing evidence for a high rate of both overt and hidden epileptic seizures in AD.5 In addition, several animal studies have indicated that in AD mouse-models frequent non-convulsive seizures contribute to the impairment of memory functions.6 Based on these studies, epileptic hyperexcitation appears to have an impact on the development and progression of AD in animal models.7 The primarily affected brain structure in AD is the hippocampus, the key organ of mesio-temporal epilepsy and the most epileptogenic formation of the brain.8
Apparently, from among the non-convulsive epileptic seizures, complex partial ones are the most common in AD.5 However, their actual significance and prevalence has remained unclear for three main reasons:
Standard scalp EEG (lasting for 30 minutes) performed in the waking state has relatively low yield for capturing seizures;12 sleep enhances the chance of detecting interictal epileptiform signs.13-15 Long-term EEG and video-EEG monitoring have been shown to significantly increase the likelihood of capturing seizures both in animal6 and in human AD studies.16 Recent findings identified strong correlation (r: 0.98) between the length of EEG recording and the probability of detecting epileptiform EEG signals in dementia patients.17
In 2014, in the framework of the National Brain Research Program in Hungary, we have launched a 4-year long investigation to define the prevalence of epileptic seizures in AD, and to find the best diagnostic tool for seizure detection in this patient population.
In the present pilot study we report on three patients representing the fascinating spectrum of different links between epilepsy and dementia/AD. We aim to show the crucial role of long-term EEG/video-EEG monitoring in assessing the significance of epilepsy in dementia.
Each patient was admitted to the Neurology Department of the National Institute of Clinical Neurosciences, Budapest, Hungary with a prior diagnosis of probable AD. Medical, neurological and psychiatric examinations, as well as blood and urine tests were carried out.
Computer tomography (CT) and/or magnetic resonance imaging (MRI) were performed.
For evaluating the patients’ cognitive status we used the mini-mental state examination (MMSE) because it is a simple and ubiquitous score for following the progression of dementia, and the Addenbrooke’s Cognitive Examination (ACE) because it is sensitive in assessing the components of cognition.18 Anxiety and depression are frequent comorbidities in dementia having major impact on the patients’ cognitive performance. Therefore, we used the Spielberger State and Trait Anxiety Inventory (STAI) and the Beck Depression Inventory II (BDI-II) to assess their presence and severity.19-20
We performed standard EEG (BrainAmp Standard Electroencephalograph 11-467) and 24-hour EEG or video EEG-monitoring (Micromed Morpheus 34 channel Polysomnograph; System 98 Micromed, respectively) in each patient.
Based on our findings, we reassessed the prior diagnosis of dementia using the revised NINCDS-ADRDA criteria.21
We obtained the permission of the local ethical committee and the patients’/caregivers’ informed consent for all tests performed.
Patient 1
The cognitive symptoms of the 70-year-old female patient were first noticed in 2009. At that time she was hospitalized and her occasional confusion, memory impairment, psychomotor slowing and alternating short periods of agitation and lethargy were investigated. Her past medical history was unremarkable and she did not take any regular medication. Routine blood tests and standard EEG were normal. MRI and MR angiography were evaluated as normal with no hippocampal or fronto-temporal atrophy. Her MMSE scored 25 points indicating mild dementia. She was diagnosed with probable AD and 10mg/day donepezil was initiated.
In 2010 she was admitted to our neurology department because of suffering two generalized tonic-clonic seizures first ever in her life. The CT brain-scan revealed mild atrophy bilaterally in the temporal, frontal and parietal regions. The neuropsychological tests suggested severe deficit in episodic memory with partially preserved semantic memory, impairment in visuo-constructive-, and language functions (ACE:65) as well as moderate cognitive decline with no anxiety (STAI: 24,26) or mood change (BDI-II: 5). This time her MMSE scored 23 points suggesting a slow progression of her cognitive deterioration. Standard EEG showed 7-8Hz background activity with occasionally superimposed low amplitude 20-24Hz fast rhythm in the frontal region. We have recorded several runs of rhythmic theta (3-5Hz) activity of 3-4s duration and occasional sharp-waves with phase-reversal at the T5 electrode. The electro-clinical presentation was suggestive of partial epilepsy with secondarily generalized tonic-clonic seizures. Based on the diagnosis of epilepsy and the marked cognitive fluctuations we suspected non-convulsive status and/or unnoticed seizures, therefore we performed 24-hour video-EEG monitoring. The recording showed frequent rhythmic theta series over the left temporal region, both during sleep and wakefulness, lasting for 5-30s (Figure 1). These theta runs were consistent with electrographic seizures and they appeared repeatedly with only a few minutes between them. The whole EEG presentation suggested non-convulsive status epilepticus.22 During the electrographic seizures, no motor or other overt clinical seizure manifestations were seen on the video; but a lethargic, inactive and disoriented state of the patient.

Figure 1 24-hour long EEG monitoring of patient 1 revealed rhythmic theta series over the left temporal region in wakefulness lasting for 12s (black arrow). 0.3Hz high pass, 70Hz low pass filter.
We started antiepileptic treatment- 10mg clobazam and 200mg carbamazepine twice a day. Based on repeated standard EEGs her non-convulsive status appeared to have resolved, however, we could not exclude the recurrence of occasional electrographic seizures or even non-convulsive status epilepticus by this method. Later, at home, her orientation and psychomotor performance somewhat improved, based on the report of her caregiver. However, her cognitive decline steadily progressed until her death, which occurred in 2014. Her last MMSE, which was taken in 2012 (16 months before she died), scored 20 points. She deceased due to sudden cardiac death while sitting in her dining room. She severely deteriorated by then, she was unable to speak, to perform personal care or to recognize her husband; she could only sing some childhood melodies.
Our patient matched the core diagnostic criteria of AD and no exclusion criteria were present, however the MRI scan did not show the characteristic pattern of atrophy. We were not able to perform CSF or PET examinations,21 and to our knowledge, no autopsy was carried out, thus, we could not make a definitive diagnosis. Although the clinical presentation might easily be interpreted as possible AD associated to seizures, the option remained that epilepsy/non-convulsive status was the only cause of her cognitive decline.23
Patient 2
The cognitive deterioration of the 91-year-old female started insidiously in 2012. She had daily recurring confusional episodes and steadily worsening memory. Her caregiver reported frequent loss of contact, speech arrests and significant deterioration of her cognitive performance. Sometimes she was completely oriented, was able to recognize the family members and to appropriately answer questions, but other times she could not even speak. Based on her cognitive decline she was diagnosed with senile dementia in 2013. She was admitted to our department in 2014 because of the suspicion of non-convulsive epileptic seizures underlying her variable cognitive performance. Her blood tests were normal. Her brain MRI scans indicated severe bitemporal atrophy with diffuse, slurred subcortical, basal ganglia and brainstem vascular lacunae. Her MMSE was 18 points, suggesting moderate cognitive decline; impairment in verbal fluency and in visuo-spatial functions was revealed (ACE: 37) with low mood (BDI-II: 16) and severe anxiety (STAI: 68, 72). She stayed in bed and slept the whole day. She had urinary incontinence. Carotid and vertebral color duplex ultrasonography (US) showed plaques in the right carotid bifurcation and in the left internal carotid artery. Transthoracal echocardiography revealed mild, concentric hypertrophy of the left ventricle without wall movement disturbances.
Standard EEG showed diffusely slowed, irregular background activity of 6-7Hz frequency and 30-40µV amplitude with the superposition of low-amplitude, diffuse, irregular fast rhythms. We recorded slow sharp components of 60-70µV in the right temporal region with phase-reversal at the T6 electrode. Occasionally, similar slow sharp elements were seen over the left temporal region. We performed 24-hour EEG to exclude ictal activity underlying her daytime cognitive fluctuations. It showed left temporal, 6-10s long, irregular delta and theta series in wakefulness and in superficial sleep with rare sharp-waves, consistent with interictal epileptiform activity. The last third of sleep recording revealed a definitive sharp-wave focus in the left temporal region (Figure 2).
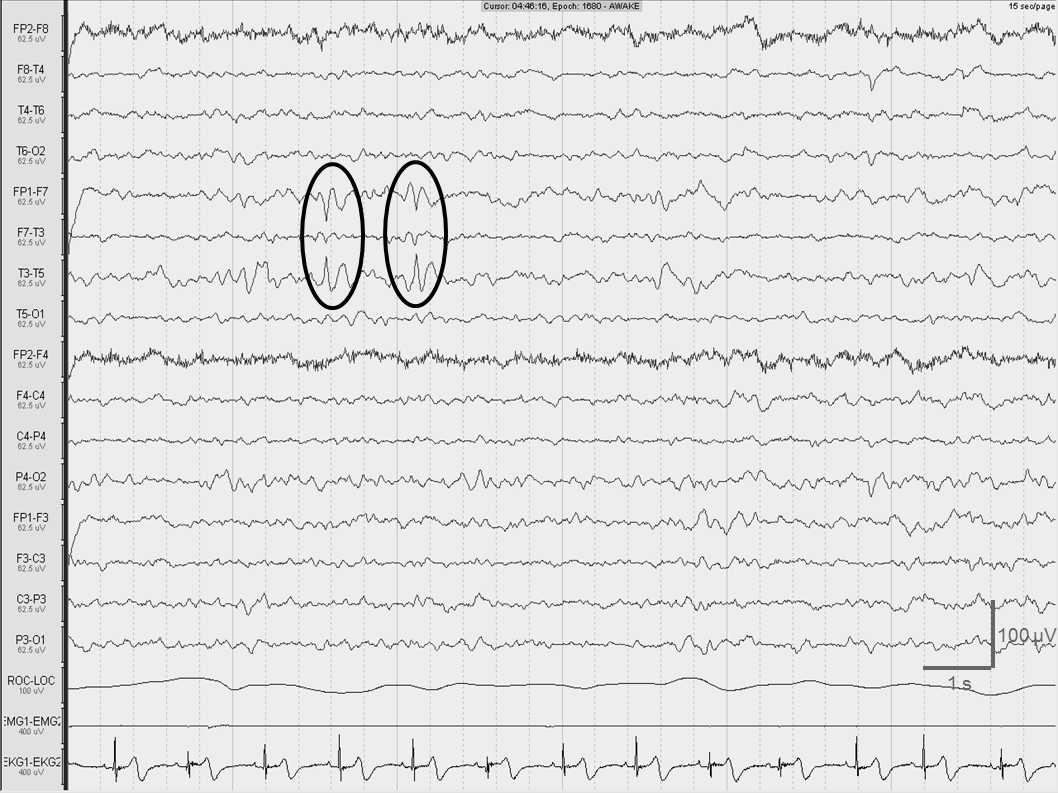
Figure 2 Definitive sharp-wave focus in the left temporal region has been identified in wakefulness by 24-hour long EEG recording of patient 2 (black circles). 0.3Hz high pass, 70Hz low pass filter.
Because of the carotid US and echocardiography findings we started acetylsalicylic acid 100mg/day; and for antiepileptic medication 500mg levetiracetam twice a day.
Six days later the control EEG showed less frequent left temporal theta and delta series but more prevalent sharp-waves in the left temporal channels, while the behavior of the patient did not change.
We diagnosed mixed type dementia (AD and contributing subcortical lesions) and concluded that the temporal lobe epileptic activity might have compromised her cognitive functions. Two months later her caregiver reported increased daily activity and enhanced aggressive behavior. The caregiver attributed this change to the antiepileptic treatment, therefore terminated levetiracetam without informing the patient’s neurologist. The patient condition is slowly deteriorating, the variability of her cognitive performance is less prominent, but she is permanently disoriented in time and space.
Patient 3
The 68-year-old male, who was a teacher of physics in high school, was hospitalized in 2012 because of progressive memory-loss for about 3 years. His past medical history was unremarkable, he did not take any regular medication and his laboratory findings were normal. At that time, his brain MRI scan showed bilateral fronto-temporal atrophy, standard EEG revealed diffuse slow background activity. He was diagnosed with probable AD based on the progressive memory loss and the typical MRI findings. Acetylsalicylic acid (100mg), atorvastatin (20mg) and vinpocetine (30mg) was initiated. We admitted him in 2014 because his caregiver reported significant fluctuations in his cognitive performance. Sometimes he was able to remember the names of his family and important dates; sometimes he was confused and disoriented. At admission we did not find any focal neurological symptom, however, we noticed abrupt changes in his cognitive performance. Routine blood checks were normal. His MRI revealed bilateral fronto-temporal atrophy, with emphasis on the temporal lobes and significant volume loss of the hippocampi. The neuropsychological tests showed severe cognitive decline; his MMSE score was 13. The impairment of his short-term memory and visuo-spatial skills was compatible with the AD-like dementia-pattern (ACE: 36) with minimal depression (BDI II: 13) and low-level anxiety (STAI: 31, 33). We confirmed the earlier diagnosis of probable AD and started 10mg donepezil and 20mg memantine once a day. Although his standard EEG was normal, because of the short-term variability of his cognitive performance we carried out 24-hour EEG for screening. It revealed irregular, 4-6 Hz diffusely slowed background activity with rare, left temporal sharp waves and occasional left temporal 6-15 s long rhythmic theta runs both in wakefulness and in sleep, consistent with electrographic seizures (Figure 3). We added 500mg levetiracetam twice a day to his anti-dementia medication. One week later his home caregiver reported improvement in his cognition and memory function, but he started to deteriorate again after 3 weeks. Because of the worsening the caregiver suspended the antiepileptic treatment. The patient was lost to follow up.
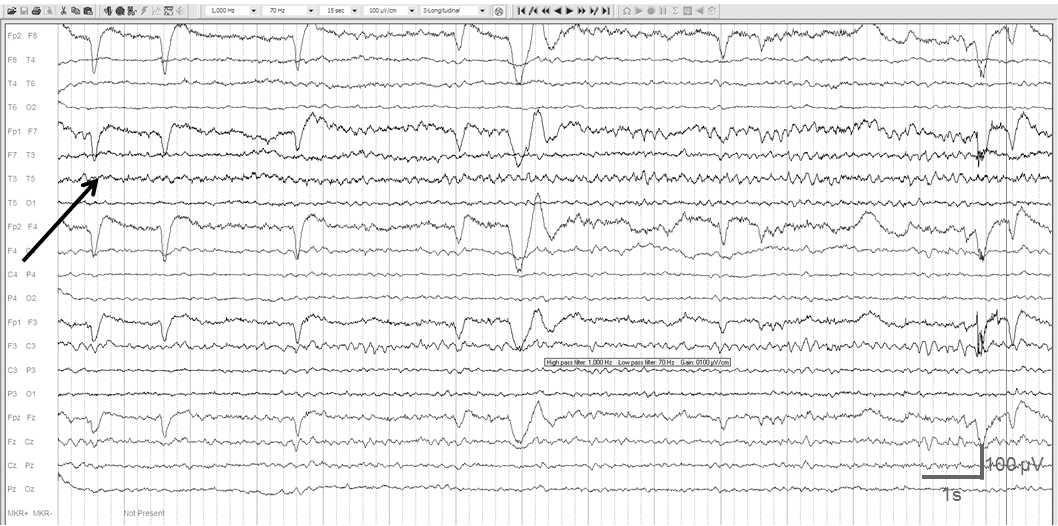
Figure 3 24-hour long EEG recording of patient 3 showed left temporal 13s long rhythmic theta runs in wakefulness (black arrow). 1Hz high pass, 70 Hz low pass filter.
Based on the clinical presentation supported by the MRI changes, it is likely that our patient suffered from AD, which was associated with left temporal epileptiform activity with no clinical signs of epilepsy. Long-term EEG was performed because of the short-term variability of his cognitive performance which was suggestive of non-convulsive epileptic seizures. Indeed, the EEG revealed interictal epileptiform activity and electrographic seizures in the left temporal region.
In this pilot observational study, we aimed to explore the link between cognitive decline and epilepsy. We highlight different types of this apparently mutual relationship and emphasize the role of long-term EEG in the diagnosis of epilepsy in demented patients.
We could not verify the definitive diagnosis of Patient 1. She could have AD complicated by temporal lobe epilepsy, or alternatively; she could have temporal lobe epilepsy only characterized by frequent electrographic seizures and non-convulsive status epilepticus with no primary dementia/AD.24
Patient 2 represents a mixed type- AD and subcortical vascular- dementia compromised by temporal lobe epilepsy. The relation of the neurodegenerative process and her epilepsy is not clear.
Patient 3 is a fascinating example of probable AD associated with left temporal electrographic seizures and left temporal interictal epileptiform activity, certainly contributing to his cognitive decline.
Our cases highlight the challenging nature of the differential-diagnosis of neuro-cognitive disorders. AD is easily confounded by multi-infarct dementia and fronto-temporal dementia. Lewy body dementia,25 neuro-infections,26 depression,27 autoimmune encephalopathies28 and normal pressure hydrocephalus29 are easily misdiagnosed for AD. Not recognizing the treatable NCD forms may lead to missed treatment on one hand and unnecessary AD treatment on the other. Some drugs might compromise the patient’s cognitive status in non-AD dementia forms: e.g. memantine may cause exacerbation of Lewy body’s dementia.30 Adequate neuro-imaging and appropriate neuropsychological tests help the differentiation of various dementia types. Because epileptic seizures may impair cognitive functions24,31 and complex partial seizures could simulate dementia-like changes,32 cognitive decline may be aggravated by unrecognized epilepsy. Non-convulsive seizures are especially hard to differentiate from NCD, and might even be misdiagnosed as primary dementia.33 Untreated epilepsy may lead to severe complications, like status epilepticus and injuries. In addition, some drugs used for the treatment of AD could provoke seizures.34
The diagnosis of epilepsy in the elderly might be difficult because age modifies the occurrence of interictal epileptiform activity.35 In elderly subjects, compared to younger age groups interictal epileptic discharges showed a lower rate on standard EEG (p<0.01), but higher propensity for spiking during slow wave sleep was detected.36 Consequently, in elderly patients sleep EEG might enhance the identification of epileptiform activity.
There is increasing evidence for the high prevalence of epileptic seizures in AD, especially in early onset familiar cases.11 Temporal lobe epilepsy is frequent in the elderly and in AD,37 all of our three patients suffered from temporal lobe epilepsy. Detection of the epileptic events may be hard in confused dementia patients, while seizures may have a strong impact on cognitive status and disease progression.38
It is confirmed that antiepileptic treatment may improve memory functions and behavioral alterations both in animal models39 and in human studies of AD.40 Our pilot study involved too few patients to be able to draw any conclusion in this respect.
There is an urgent need to enhance the diagnostic accuracy for the early detection of seizures in AD to identify epilepsy as a treatment target. Standard scalp EEG cannot sufficiently detect hidden temporal lobe epileptiform activity.12 We suggest that in the dementia patients long-term EEG recordings containing slow wave sleep should be performed to facilitate the detection of interictal or ictal events. Because epileptiform discharges occurring in the temporo-basal or mesial temporal regions are far from the scalp, even the use of semi-invasive EEG techniques (foramen ovale electrode) may be justified in properly selected cases, in order to improve treatment and outcome in Alzheimer’s disease.
The present study showed the pharmacological potential of the ethanolic extract of Neem bark. Our findings demonstrated that the F-EtOAc, obtained after saponification of EtCNeem, showed to be rich in phenolic and flavonoid compounds with antioxidant potential, as well as a nontoxic.
Our research was supported by the National Brain Research Program (KTIA_NAP_13-1-2013-0001) and MET Hungary Ltd. Authors have no conflict of interest and nothing to disclose.
None.

©2016 Horváth, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.