Journal of
eISSN: 2373-6410


Review Article Volume 9 Issue 2
Department of Medicine Zulia University and Institute of Clinical Neuroscience, Venezuela
Correspondence: Orlando J Castejón. Institute of Biological Research "Drs. Orlando Castejón and Haydee Viloria de Castejón". School of Medicine. University of Zulia. Section 526. Maracaibo, Venezuela
Received: February 22, 2019 | Published: April 24, 2019
Citation: Castejón OJ. Morphopathological changes of dendrites in experimental animals and in human nervous diseases. J Neurol Stroke. 2019;9(2):98-104. DOI: 10.15406/jnsk.2019.09.00356
The present review describes the pathological changes of shaft dendrites in most central nervous diseases. We have illustrated most pathological changes using cortical biopsies of patients with congenital hydrocephalus, severe and complicated traumatic brain injuries, and brain tumors. Swollen and beaded dendrites exhibit fragmentation of limiting plasma membrane, cytomembranes and cytoskeletal structures. The swollen dendrites show vacuolization, dense residual bodies, enlarged rough and smooth endoplasmic reticulum, edematous clear and dark mitochondria. The multifactorial processes associated with brain edema and brain ischemia, such as calcium overload, activation of calcium-dependent proteolitic enzymes, protein aggregation, glutamate-induced neurotoxicity, release of lysosomal enzymes, deficit of ATP, stress oxidative and lipid peroxidation have been considered in relation with pathological dendritic changes. Dendrotoxicity due to brain edema and brain ischemia seems to be the fundamental pathogenetic mechanism underlying the dendritic damage.
Keywords: dendrites, brain edema, brain trauma, hydrocephalus, brain tumors, electron microscopy
Dendritic development and arborisation show aberrant or anomalous patterns in aging process and in various central nervous system diseases, such as brain trauma, schizophrenia, neurodegenerative diseases, epilepsy, malnutrition in developing brain, infections, mental retardation, hydrocephalus, cerebral ischemia, and exposure to alcohol and other toxins.1–48 The formation of meganeurites in human neuronal storage diseases,4,8 the existence of more branched dendrites in neuronal elderly individuals,49,50 and the appearance of new dendritic growths in Alzheimer disease,1 reveal that the adult human neuronal system appears capable of responding to various stimulus, and exhibits the potential to modify existing neuronal connections. Abnormal dendritic development and dendritic spine “dysgenesis” have been reported in mental retardation,28,51,52 and severe protein-calorie malnutrition.27 Aberrant dendritic growth and aberrant patterns of spine morphology have been reported by Machado-Salas53 in Bourneville’s disease. Marked atrophy of basal and apical dendrites of neurons of layer 3 and 5 of cerebral cortex in Tay-Sachs disease was reported by Takashima et al.12 Loss of Purkinje cell spines, cactus-like thickenings and atrophy of Purkinje cell dendrites may be found in Menke´s disease,51 and in experimental encephalopathy induced by chronic application of valproate.36 Abnormalities of dendritic arborization have been observed by light microscopy in a variety of cerebral malformation, such as microgiria and lisencephaly.10
In epilepsy a wide spectrum of dendritic pathology has been recognized, such as loss of dendritic spine and development of nodular or fusiform enlargements along the dendritic shafts.55–57 Dendritic abnormalities have been also described in normal aging and various dementias.5,50 Normal elderly individuals have longer and more branched dendrites than younger and senile dementia patients.4,58,59Abnormal dendrites have been also found in Huntington´s disease.60 Age-related regulation of dendritic endocytosis was reported by Blanpied et al.61 Castejón & Arismendi23 described swollen and beaded dendrites, disrupted of limiting plasma membrane and cytoskeletal structures in the human edematous cerebral cortex associated to brain trauma, congenital malformations, and brain tumors. Works et al.62 reported age-dependent dendritic atrophy of basilar dendrites in the rat nucleus magnocellularis related with loss of cholinergic innervation. Vega et al.63 described increased dendritic length, and decreased density of synaptic spines in the prefrontal cortex of rat with renovascular hypertension. Allred and Jones (2004) found dendritic structural plasticity after unilateral ischemic damage of rat sensory motor cortex. Wedzony et al.64 reported diminished length of basilar dendrites of prefrontal pyramidal neurons in adult rats after blockade of NMDA receptors in the postnatal period. Rensing et al.65 (described dendritic swelling and loss of spines during electrographic seizures induced by 4-aminopyridine in transgenic mice. Peyghambari et al.46 found a significant reduction in the length of most dendrites in the axotomized motoneurons of the spinal cord in newborn rats. Radley et al.66 encountered reversible apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Brown et al.67 also found remodelling of apical dendrites, atrophy of distal branches, and sparing of proximal branches induced by stress in medial prefrontal cortex. Flores et al.47 reported decreased length of basilar dendrites in post-puberal rats after nenonatal excitotoxis lesions of the ventral hippocampus. Zaja-Millatovics et al.68 demonstrated shortened dendritic length of neostriatal medium spiny neurons in Parkinson disease. Ishikura et al.69 described dendritic atrophy in prion disease. Dierssen & Ramakers70 emphasized the dendritic pathology in mental retardation from the genetic point of view. Shimada et al.71 studying a model of cerebral degeneration, the ageing SAMP10 mouse, described age- related dendritic retraction in the entire cerebral cortex and olfactory bulb. Brief exposure to excitotoxic agonists can result in substantial loss of the microtubule-associated protein MAP2 from neuronal dendrites, and accumulation in neuronal somata. A possible mechanism underling MAP2 loss is the activation of the calcium-dependent protease calpain by excessive dendritic Ca2+-loading.72
Dlugos73 reported smooth endoplasmic reticulum dilation and degeneration in Purkinje neuron dendrites of aging ethanol-fed female rats. Baloyannis et al.74 describe substantial alteration of dendritic arborisation in the acoustic cortex in Alzheimer’s disease.
In instances of CNS injury or disease, increased concentrations of extracellular glutamate can result in the over-activation of ionotropic glutamate receptors and trigger neuronal cell death (termed excitotoxicity). Two early hallmarks of such neuronal toxicity are mitochondrial dysfunction (depolarization, decreased ATP synthesis, structural collapse and potential opening of the permeability transition pore) and the formation of focal swellings (also termed varicosities/beads) along the length of the dendrites.75
Dlugos73 reported dilation of the smooth endoplasmic reticulum (SER), and the formation of degenerating bodies within Purkinje neuron dendrites of aging ethanol-fed female rats, According to this Author, dilation of the SER and the formation of degenerating bodies may be a predictor of dendritic regression. Shansky & Morrison76 reviewed the stress-induced dendritic remodeling in the medial prefrontal cortex (mPFC), with particular focus on new findings that illuminate modulators of these effects. Compression instantly twisted the microtubules and deformed the membrane contour of dendritic trunks, and immediately reduced dendritic spines on the entire dendritic arbor. Liu et al.77reviewed the dendritic changes that have been recorded in neurodegenerative processes including those occurring in development, ageing and diseases. The findings suggest that dendritic pathology is an early sign in disease and underline the importance of synapto-dendritic structure, providing new insights into therapeutic strategies.
Deng et al.78 made the characterization of dendritic morphology and neurotransmitter phenotype of thoracic descending propriospinal neurons after complete spinal cord transection and GDNF treatment. Deng & Reiner78 found reductions in dendritic branching and thalamostriatal input in cholinergic interneurons in the Q140 knockin mouse model of Huntington's disease.
Tau protein in dendrites and synapses has been recently implicated in synaptic degeneration and neuronal malfunction. Chronic stress, a well-known inducer of neuronal/synaptic atrophy, triggers hyperphosphorylation of Tau protein and cognitive deficits. Exposure to chronic stress resulted in atrophy of apical dendrites and spine loss in the prefrontal cortex (PFC) neurons. Tau may exert its effects through synaptic mitochondria.79 Nava et al.80 described the temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. These authors found significant atrophy of apical dendrites at 1day, which was prevented by chronic desipramine, and at 14days after stress exposure.
Saberi et al.81 reported dendritic-like aggregates in the motor cortex that co-localized with pTDP-43 and their repeat RNAs dipeptide repeat proteins (DPRs) in amyotrophic lateral sclerosis.
In the present review we describe the structural changes of dendrites in different esperimental and human central nervous system diseases, and we analyze at submicroscopic level the dendritic morphological changes of nerve cells in the edematous human cerebral cortex associated to congenital hydrocephalus, brain trauma, and brain tumors in an attempt to provide better insight on the pathological changes induced by these distinct nosological entities, and the associated brain ischemia.
Submicroscopic changes of dendrites in congenital hydrocephalus.
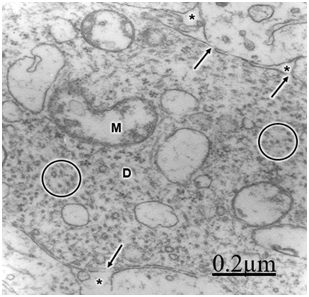
The immature hydrocephalic cerebral cortex neuropil in neonate patients with congenital hydrocephalus shows irregularly beaded shaped, and swollen and vacuolated dendritic processes with elongated and dark mitochondria. Most patients with congenital hydrocephalus exhibit lamellipodic and filopodic dendritic processes, and endocytic vesicle formation at the limiting plasma membrane,82 These dendrites exhibit mushroom, stubby and filopodic types of dendritic spines making asymmetric synaptic junctions (Figure 1).83 Some dendritic processes show fragmented plasma membrane in areas of severe brain hydrocephalic edema (Figure 2).
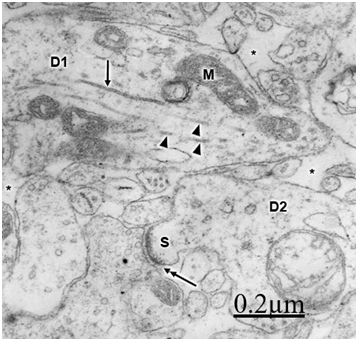
Mc Allister et al.84 reported dendritic varicosities and spine loss as the most striking dendritic alterations in experimental induced hydrocephalus in newborn rats. Harris et al.85 found a decreased in the total length of dendritic tree in the infant H-TX rats. Hydropic dendritic deterioration has been reported in feline-infantile hydrocephalus by Kreibel & McAllister.30
Dendrite pathology in human traumatic brain injuries
In patients with traumatic brain injuries exhibiting contusions and associated subdural or extradural hematoma or hygroma, varicose swollen dendrites with fragmented plasma membranes, disruption of cytoskeletal structures characterized by disintegrated microtubules and neurofilaments, electron lucid and vacuolated dendroplasm, enlarged rough and smooth endoplasmic reticulum, partial loss of dendritic spines, increased vesicular transport of microvesicles, dense round and elongated inclusion bodies, and complex or clathrin-coated vesicles are observed (Figure 2) (Figure 3).
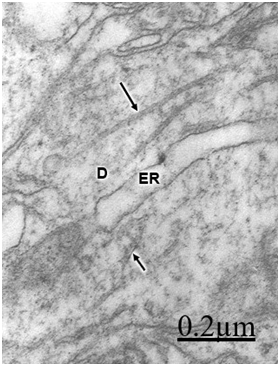
Dendritic angulations, and nodular or segmentary dendritic swelling were earlier reported by Vaquero et al.55, Gallyas & Zoltay13 and Swann et al.31 in human epileptic dendrites. According to Vaquero et al.,55 the nodular dendritic swellings are due to alteration in the microtubular arrangement. Vacuolated dendrites inducing hydropic deterioration and degeneration of dendrites have been reported by Goldstein et al.6 in rat central nervous system after ethanol consumption, by Posmantur et al.86 after traumatic brain injury in rats, and by Sobaniec-Lotowska36 in rat experimental encephalopathy induced by valproate. Saito et al.87 found calcium accumulation in swollen dendrites following cerebral ischemia and traumatic brain injury. Gallyas & Zoltay13 considered that in cases of head injury, the beaded appearance of dendritic and axonal processes indicates an advanced stage of morphopathological damage. In addition, some neurons exposed to hypothermia, NMDA or ionophore also developed beaded dendrites (Emery and Lucas, 1995). Focal dendritic swelling was observed by Ferrer et al. (1998) in mucopolysaccharidoses types I, II and III. The focal swelling of dendrites is apparently similar to that observed in axonal processes also due to destruction of cytoskeletal network.88,89 Swollen and beaded dendrites have been widely reported in a large variety of pathological entities. Dendritic swelling was observed in stroke-prone spontaneously hypertensive rats,5 following intrathecal infusion of N-methyl-D-aspartate,90,91 in rats with neuroleptic-induced dyskinesias,92 and in rat brain during acute focal ischemia.32 Swann et al.56 postulated an ongoing excitotoxic injury of dendrites (dendrotoxicity) produced by excessive release of glutamate especially during seizures. In brain trauma there is also glutamate-induced citotoxicity,93 which supports Swann et al.94 hypothesis. According to Hasbani et al.94 the postsynaptic neuronal dendrite is selectively vulnerable to hypoxic-ischemic brain injury and glutamate receptor overactivation. Sodium, chloride, and water entry contribute acutely to excitoxicity dendritic injury, and calcium entry through NMDA receptors results in lasting structural changes in damaged dendrites. Lately Hasbani et al.94 expressed that in cerebral ischemia, neurons exposed to NMDA, kainite or oxygen-glucose deprivation suffer dendritic beading and lost of dendritic spines.
Lee et al.95 point out that Ca++-activated degradation of cytoskeletal proteins appears to be an early and important component of the post-ischemic response in hippocampal neurons, which can contribute to neuronal death. According to Tomimoto & Yanagihara,97 the disintegration of microtubules and the resulting disruption of dendritic transport may contribute to subsequent development of delayed neuronal death. According Hayes et al.98 the traumatic brain injuries produce a widespread derangement to the neuronal cytoskeleton.
The molecular mechanism inducing the disintegration of cytoskeletal structures in traumatic brain injury could be due to loss of cytoskeletal proteins and microtubule associated protein 2 (MAP2), possibly by calpain-mediated proteolysis.86,99 Brain contusions also induce loss of both, MAP2 and neurogranin immunoreactivity.100
Mild and repetitive brain injuries may trigger cytoskeletal alterations related to neuronal degeneration and abnormal behaviour.101 Cytoskeletal disruption is a key pathological feature of Alzheimer’s disease, characterized by dendritic degeneration.5,102
Ultrastructural abnormalities of dendrites with damage of endoplasmic reticulum, mitochondrial lesion and disintegration of microtubules have been observed after chronic administration of valproate.36 Similar dendritic changes have been recently observed after fluid perfusion injury.103
Our findings suggest that anoxia e ischemia are the major pathogenetic mechanisms of dendritic swelling in the edematous human cerebral cortex associated to brain trauma, tumours and congenital malformations.83 Our observations on dendritic abnormalities in brain traumatic injuries revealed predominant beaded shape of swollen dendrites in comparison with those seen in brain malformations and tumors. The beaded dendrites exhibit disintegrated microtubules and microfilaments mainly at the dendritic varicosities. Derangement of dendritic cytoskeletal structures, mainly fragmentation and disintegration of microtubules and neurofilaments, are due to multifactorial factors, such as the shear stress induced by the traumatic agent, mitochondrial swelling, anoxic-ischemic condition of brain tissue, and protease activation.24,87
In relationship with the damage of the limiting plasma membrane and the dendritic cytomembranes, such as mitochondrial, rough and smooth endoplasmic reticulum, lysosomal and Golgi membranes, could be due to increased permeability of lysosomes and release of acid and neutral proteases,104 interruption of dendritic transport,97 calpain-mediated spectrin breakdown, free radical release and lipid peroxidation,105–107 delayed phospholipid degradation by phospholipase activation,108 disruption of cytoskeletal structures, mitochondrial abnormalities and impaired production of ATP, elevation of intracellular calcium,87,99 activation of calcium-dependent proteolitic enzymes,93 glutamate-induced neurotoxicity,31,107,110 protein aggregation after brain ischemia and reperfusion,111,112 intensity of shear forces in brain traumatic injury, increased intracranial pressure in moderate and severe edema.23,24 and release of lysosomal enzymes.113
Dendritic abnormalities in brain tumors
In relationship with the alteration of dendritic processes in brain tumors, such as in cystic craniopharyngioma and ependymoma, we have observed swollen dendrites with a granular proteinaceous aggregation in the dendroplasm, vacuolated rough and smooth endoplasmic reticulum canaliculi, dark and clear swollen mitochondria, disintegrated neurofilaments, scarce amount or absent of microtubules, presence of clathrin-coated vesicles and myelin-like figures24 (Figure 5). Swelling of dendrites with disarray of microtubules and neurofilaments and changes of surface morphology of dendritic spines were earlier reported by Spacek38 in epitumorous cerebral cortex.114,115
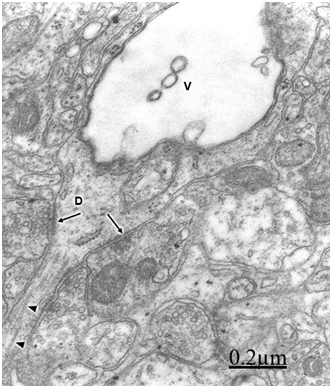
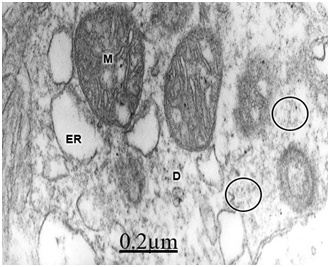
Swollen and beaded dendrites exhibit fragmentation of limiting plasma membrane, cytomembranes and cytoskeletal structures. The swollen dendrites show vacuolization, dense residual bodies, enlarged rough and smooth endoplasmic reticulum, and edematous clear and dark mitochondria. The multifactorial processes associated with brain edema and brain ischemia, such as calcium overload, activation of calcium-dependent proteolitic enzymes, protein aggregation, glutamate-induced neurotoxicity, release of lysosomal enzymes, deficit of ATP, stress oxidative and lipid peroxidation have been considered in relation with pathological dendritic changes. Dendrotoxicity due to brain edema and brain ischemia seems to be the fundamental pathogenetic mechanism underlying the dendritic damage.
None.
Author Declare there is no conflict so interest.

©2019 Castejón. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.