Journal of
eISSN: 2373-6453


Allogeneic stem cell transplantation of hematopoietic stem cells (HSCs) resistant to HIV-1 (CCR5 null cells)using a regimen to improve homing, engraftment, and preferential cord chimerism of umbilical cord blood (UCB) cells homozygous for CCR5∆32 mutation (UCBCCR5∆32/∆32) favors utilization of prostaglandin E2 (PGE2)-mediated mechanisms to facilitate allogeneic transplantation. Since PGE2 has additional capacity to down-regulate CCR5 expression, this could enable dual effect
Such treatment has capacity not only to improve engraftment of UCBCCR5∆32/wt cells heterozygous for the CCR5∆32 mutation but also prevent functional expression of the CCR5 chemokine receptor used by CCR5 (R5)-tropic HIV-1 to enter CD4+ T cells Provided that PGE2 or its more stable analogue dimethyl-PGE2(dmPGE2) has lasting effects in HSCs this could lead to significant increase of the number of treatable patients (frequency of heterozygous CCR5wt/∆32 donors in US and Central and North of Europe is at least 10 fold higher than in the case of homozygous CCR5∆32/∆32 donors). Thus, ultimate goal is to create a functional R5-tropic HIV-1-resistant immune system through the use of optimized dmPGE2-modified UCB cells heterozygous for ∆32 mutation with improved homing, engraftment, and preferential cord chimerism with further emphasis on post-transplant amelioration of the graft versus host disease (GvHD) enabled via PGE2-mediated potentiating of regulatory T (Treg) cell-mediated suppression.
Keywords: CCR5, HIV-1, HSC, UCB, PGE2, dmPGE2, GvHD, nTregs, iTregs, Tcons, cAMP, ICER
CCR5: Chemokine C-C Motif Receptor 5; HIV-1: Human Immunodeficiency Virustype-1; HSC: Hematopoietic Stem Cell; UCB: Umbilical Cord Blood; PGE2: Prostaglandin E2; dmPGE2: Dimethyl Prostaglandin E2; GvHD: Graft-Versus-Host Disease; nTregs: Naturally Occurring Regulatory CD4+CD25+ T Cells; iTregs: Inducible Tregs; Tcons: Conventional CD4+ T Cells; cAMP: Cyclic AMP; ICER: Inducible cAMP Early Repressor
Evidence for the cure of HIV infection by CCR5∆32/∆32 stem cell transplantation
The unprecedented power of the hematopoetic stem cell (HSC) transplantation of the CCR5∆32/∆32 cells resistant to HIV has been reported in 2009 to cure HIV infection in the case of leukemia patient infected with HIV (‘Berlin patient’ - Timothy Ray Brown).1 More than five years after transplantation ‘Berlin patient ‘remains HIV free without any antiretroviral treatment (ART). Another two cases control HIV infection only temporarily after HSC transplantation of bone marrow cells treated with ART.2 These two cases reported from Brigham and Women Hospital in Boston clearly demonstrated that use of bone marrow donors with CCR5∆32/∆32 cells resistant to HIV are superior to ART treatment and may purge latent reservoirs of HIV. Currently, CCR5∆32/∆32 HSCs promise sterilizing immunity against HIV. Since ‘Berlin patient’ is the first man cured of HIV infection, there is an urgent need to repeat transplantation using CCR5∆32/∆32 HSCs resistant to HIV. Importantly the ∆32 mutation at the CCR5 locus is present in Europe, with higher frequencies in the north3 and homozygous carriers of the ∆32 mutation (CCR5∆32/∆32) are resistant to HIV-1 infection of R5 strain because the mutation prevents functional expression of the CCR5 chemokine receptor used by HIV-1 to enter CD4+ T cells while heterozygous carriers have partial resistance to HIV-1 infection of R5 strain. The concept of allogeneic stem cell transplantation of the cells resistant to HIV (CCR5 null cells) using a regimen to improve and modulate engraftment of umbilical cord blood cells (UCB) homozygous for CCR5∆32 mutation favors utilization of prostaglandin E2 (PGE2)-mediated mechanisms to facilitate both allogeneic transplantation and down-regulation of CCR5 expression (critical in UCBCCR5∆32/wt cells of heterozygous donors with partial protection against HIV infection).
Inhibitory pathway of PGE2 in Tcons
An important precedent of receptor mediated cAMP formation in inducible Tregs (iTregs) is prostaglandin E2 (PGE2) synthesis.4 It has been demonstrated that iTregs express cyclooxygenase-2 (COX-2) and produce PGE2 upon differentiation, signaling through any of its four receptors – EP1, EP2, EP3, and EP4-often with opposing effects. EP2 and EP4 appear to be the most abundant in naïve cells isolated from peripheral blood and are up-regulated in response to activation. Recent studies have provided significant insights-in particular, a pathway has been described in conventional CD4+ T cells (Tcons) where signaling through EP2 or EP4, with its concomitant increase in cAMP levels, leads to PKA activation and, through an EBP50-Ezrin-PAG scaffold process
phosphorylation of the C-terminal Src kinase (Csk). Phosphorylated Csk in turn inhibits Lck-mediated phosphorylation of the TCR complex, thus inhibiting TCR signaling and T cell proliferation and function (Figure 1).
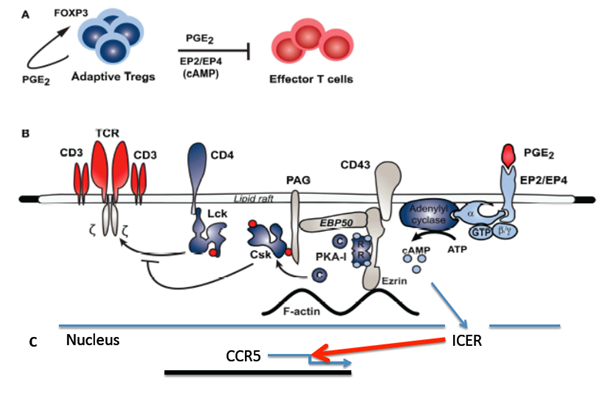
Figure 1 Model of inhibitory pathway of prostaglandin E2 (PGE2) and down regulation of CCR5 in Tcons.
PGE2 mediates Treg inhibition of effect or T cell (Tcon) function through a PKA-mediated pathway:
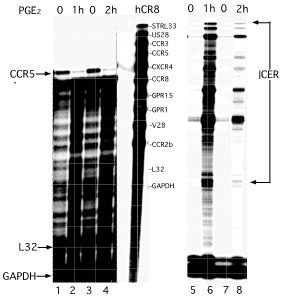
Figure 2 PGE2-mediated transcriptional attenuation of CCR5 chemokine receptor expression correlates with ICER induction.
Human monocytes were treated with PGE2 and scored for human chemokine receptor expression (left panel) in parallel with ICER expression (right panel), using RNase protection assay (Ambion). For evaluation of human chemokine receptor expression, a Riboquant hCR8 probe set was used. Levels of chemokine receptor were evaluated after PGE2 treatment (500 ng/ml final for 1h and 2h at 370 C in regular media). Levels of CCR5 are inversely related to the levels of ICER mRNA after PGE2 treatment. Templates for the analysis of hL32 and human glyceraldehyde-3-phosphate dehydrogenase housekeeping genes were included to allow assessment of total RNA levels.
Non-resolved questions of PGE2-mediated down regulation of CCR5
PGE2-mediated transcriptional attenuation of CCR5 chemokine receptor expression correlates with expression of potent transcriptional factor (TF) inducible cAMP early repressor (ICER). Our previous results indicate that PGE2 may exert direct effects on down-regulation of CCR5 expression in human peripheral blood monocytes (Figure 2). These preliminary studies suggest that in addition to ICER-mediated down regulation of interleukin 2 (IL-2) in auto reactive CD4+ conventional T cells (Tcons).5-7 ICER could also transcriptionally attenuate expression of CCR5 receptor in the presence of PGE2 (Figure 2). Therefore genome-wide analysis of the pulse treatment of dmPGE2 for Tregs and Tcons and their consequent CCR5 down-regulation along with down-regulation of other chemokines and chemokine receptors (such as CXCR4) will be informative. Furthermore, after the pulse treatment of UCB by dmPGE2 gene expression profiling may optimize an ex vivo modulation protocol and UCB engraftment, bone marrow homing, and preferential cord chimerism in heterozygousUCBCCR5∆32/wt cells, which could be analyzed in humanized mice model. Potential down-regulation of CCR5 expression by dmPGE2 in UCBs from donors heterozygous for ∆32 mutation could significantly increase probability of appropriate UCB selection for bone marrow transplant in HIV infected patients with hematologic malignancies.
Prostaglandin-modulated hematopoietic stem cell transplantation
At present, the general consensus is that ‘true’ self-renewing human HSCs are found within the CD34+ population and that engraftment of a suitably conditioned host with a sufficient number of such cells will result in long-term multi-lineage hematopoiesis.8,9 UCB cells are a valuable source of HSCs for use in allogeneic transplantation. Key advantages are easy availability and less stringent requirements for HLA matching. However, UCB contains an inherently limited HSC count associated with delayed time of engraftment, high graft failure rates and early mortality. PGE2 derivative (16, 16 dimethyl prostaglandin E2; dmPGE2) was recently identified to be a critical regulator of HSC homeostasis.10 Recent data have shown that brief ex vivo modulation with dmPGE2 could improve patient outcomes by increasing the ‘effective dose’ of HSCs with preferential long-term engraftment of the dmPGE2 treated HSCs in allogeneic transplantation.10 Moreover, it was demonstrated that conventional T cells (Tcons) could be developed in vitro into CD4+CD25+Foxp3+ inducible regulatory T cells (iTregs) with an equivalent suppressive potential as naturally occurring regulatory T cells (nTregs) by continuous polyclonal activation with anti-CD3/CD28 mAbs.4,11 During the differentiation process, the iTregs express cyclo oxygenase 2 (COX-2) and produce PGE2. Interestingly, neither resting nor activated nTregs express COX-2. The PGE2 production from iTregs can be fully suppressed by the COX inhibitor indomethacin.4 These data indicate that PGE2 plays an important role in differentiation of HSCs thus releasing stringency required for HLA-matching donors with potential recipients as well as with potential role in dominant suppressive effects of iTregs expressing COX-2 with acquired ability to produce copious amounts of PGE2 responsible for delivery of suppressive function through elevated levels of cAMP.12,13
Prostaglandin E2 (PGE2)-mediated mechanisms, which have potential to down-regulate CCR5 expression in umbilical cord blood (UCB) cells heterozygous for CCR5∆32 mutation (CCR5wt/∆32),could reduce or eliminate surface expression of CCR5 (CCR5 null cells). These processes may thus facilitate allogeneic transplantation, UCB cell-engraftment, and preferential cord chimerism in parallel with CCR5 down-regulation. Key advantages are higher frequency of heterozygous CCR5wt/∆32 donors, less stringent requirements for HLA matching, and better engraftment of the cells resistant to HIV. To eliminate the need for indefinite treatment, our ultimate goal is to create a functional HIV-resistant immune system through the use of modified HSCs with emphasis on post-transplant amelioration of GvHD enabled via potentiation of regulatory T cell (Treg) cell-mediated suppression.
None.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.