Journal of
eISSN: 2373-6453


Editorial Volume 1 Issue 2
Institute of Experimental Medicine AS CR, Czech Republic, Europe
Correspondence: Josef Bodor, Institute of Experimental Medicine AS CR, Videnska 1083, 142 20 Prague 4-Krc, Czech Republic, Europe, Tel 420 241 062 570, Fax 420 241 062 785
Received: June 06, 2014 | Published: June 7, 2014
Citation: Bodor J (2014) Evidence for the Cure of HIV Infection by CCR5Δ 32/Δ 32 Stem cell Transplantation. J Hum Virol Retrovirol 1(2): 00008. DOI: 10.15406/jhvrv.2014.01.00008
cAMP, Cyclic Adenosine Monophosphate; HSC, Hematopoetic Stem Cell; ICER, Inducible cAMP Early Repressor; CREM, Cyclic AMP Responsive Element Modulator; TFs, Transcription Factors; NFATc1, Nuclear Factor of Activated T Cell c1; nTreg, Naturally Occurring Regulatory T Cells; Tcons, Conventional CD4+ T Cells
The unprecedented power of the hematopoetic stem cell (HSC) transplantation of the CCR5∆32/∆32 cells resistant to HIV has been proven to cure HIV infection in the case of leukemic patient (‘Berlin patient’ – Timothy Ray Brown) reported two years ago.1 Since then another two cases proven to cure HIV infection after hematopoetic stem cell (HSC) transplantation of the CCR5∆32/∆32 cells were reported from Brigham and Women Hospital in Boston.2
The ∆32 mutation at the CCR5 locus was found in Europe, with higher frequencies in the north.3 Homozyous carriers of the ∆32 mutation (CCR5∆32/∆32) are resistant to HIV-1 infection because the mutation prevents functional expression of the CCR5 chemokine receptor used by HIV-1 to enter CD4+ T cells. The CCR5∆32 mutation is a good example of an advantageous allele with a well-characterized geographic distribution. The ∆32 mutation currently plays an important role in HIV resistance because heterozygous carriers have reduced susceptibility to infection and delayed onset of AIDS, while homozygous carriers are resistant to HIV infection.4 Bubonic plague was initially proposed as the selective agent, but the subsequent analysis suggested that a disease like smallpox is a more plausible candidate.5 Lucotte and Mercier6 suggested that the geographic distribution imply a Viking origin. In particular they proposed that the allele was present in Scandinavia before 1,000 to 1,200 years ago and then was carried by Vikings westward to Iceland, eastward to Russia, and southward to central and southern Europe.
The feasibility of this concept (Figure 1) is based on novel insights of the protection from GvHD by elevated levels of cAMP through binding of HIV-1 envelope protein gp120 to human regulatory T cells (Tregs) (Figure 2).8,9 Tregs represent a unique T-cell lineage endowed with the ability to effectively suppress immune responses. Therefore, approaches to modulate Treg function in vivo could provide ways to enhance or reduce immune responses and lead to novel therapies. It is known for a long time that Tregs need to be activated to exert their suppressive function on bystander conventional CD4+ T cells (Tcons). However, it has remained elusive how activation of Tregs may occur effectively, as their suppression is not restricted and their antigen specificity may be different from the cells they suppress. Earlier reports on anti-CD4-mediated tolerance and Treg activation took advantage of the HIV gp120 protein having been a high-affinity ligand for CD4 and reported that gp120 – mediated activation of naturally occurring Tregs (nTregs) through CD4 was sufficient to turn on the suppressive activity in nTregs (Figure 2).10 CD4-mediated activation depends on Lck and low levels of cyclic adenosine monophosphate (cAMP) production and can be blocked by Src family kinase inhibitors and adenylyl cyclase inhibitors. Functional analysis of the effects of gp120-mediated activation of Treg in vivo in a GvHD model demonstrated that the Treg activation by gp120 through adenylyl cyclase could abolish the rejection.8 The data on gp120 are very important in the context Treg-mediated suppression in vivo as a starting point for potential new therapies to ameliorate GvHD in the course of HSC transplantation of the cells resistant to HIV-1. In terms of link between Lck and the adenylyl cyclase leading to increased cAMP levels in Tregs this connection could explain how CD4 ligation and subsequent Lck activation increased intracellular cAMP concentration (Figure 2).
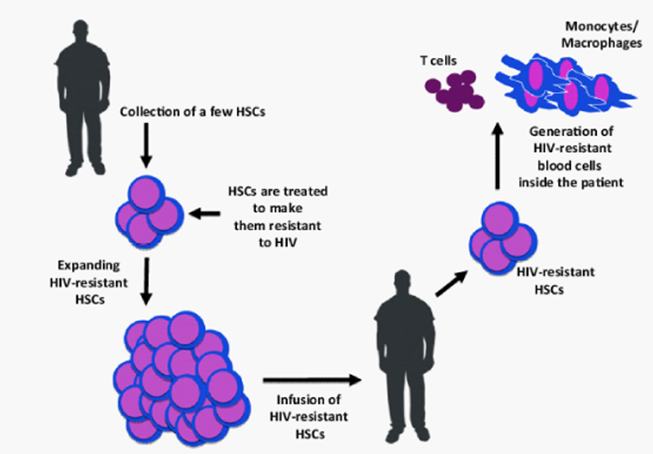
Figure 1 Hematopoetic-stem-cell based therapy for HIV disease (adopted from Kiem et al.7
Long lived, self-renewing, multilineage hematopoetic stem cells (HSCs) selected such that their progeny resist HIV-1 infection (such as HSCs from donor harboring CCR5∆32 mutation). The host could thereafter be repopulated with a hematopoetic system (including CD4+ T cells and myeloid targets for HIV) that is resistant to the replication and spread of HIV.
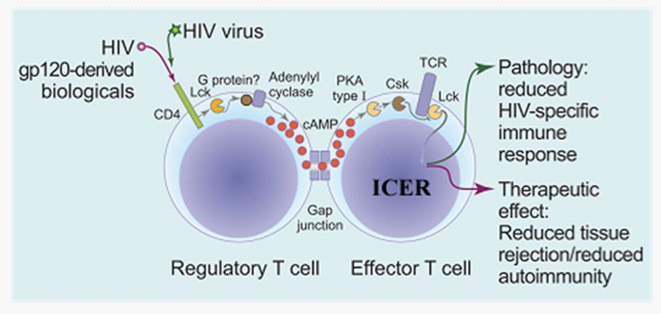
Figure 2 A schematic representation of regulatory T cell immunosuppression by cAMP following gp120 ligation of CD4 (adopted from Tasken.9
Upon triggering of CD4 on regulatory T cells by gp120 protein or possibly gp120 derived agonists, Lck becomes active and turns on cAMP production by adenylyl cyclase possibly through interaction with a G protein. cAMP is transferred from regulatory T cells to effector T cells through cell-to-cell contacts called GAP junctions that allow diffusion of small molecules down the concentration gradient and into effector T cells. Once inside effector cells, cAMP inhibits immune function through PKA-Csk inhibitory pathway that turns off T-cell activation proximally under the T-cell receptor in parallel with induction of potent inhibitor of cAMP-mediated transcription ICER (inducible cAMP early repressor). This could ameliorate graft versus host disease and lead to reduced tissue rejection and/or autoimmunity.
cAMP and ICER: A model of nTreg -mediated suppression. Since cAMP induces inducible cAMP early repressor (ICER) it was hypothesized that ICER as a potent transcriptional repressor plays an important role in nTreg cell-mediated suppression.12 ICER is generated by use of an alternative downstream promoter in the gene encoding cyclic AMP responsive element modulator (CREM).13 Inter alia, during nTreg-mediated suppression we have shown that ICER preferentially inhibits the production of IL-2, an essential growth factor for auto-reactive Tcons.14 We have recently shown that the transcription factors (TFs) ICER and nuclear factor of activated T cell c1 (NFATc1) are decisively involved in the suppression of CD4+ T cells by nTregs.15 Deficiency in these TFs led to a resistance of CD4+ T cells against nTreg cell-mediated suppression.16 Based on these data, we have proposed a model of nTreg cell-mediated suppression of Tcons through the transfer of cAMP (Figure 2) adopted from Tasken9 and Bodor et al.17 Thus in order to enable HSC transplantation of the cells resistant to HIV creation of database of bone marrow donors homozygous for CCR5∆32 mutation would be highly desirable. Secondly, based on detailed knowledge of cAMP-mediated suppression of alloreactive Tcons using Tregs harboring ∆32 mutation we should strive to improve Treg immunosuppression and optimize capacity of gp120 to ameliorate GvHD.
None.
None.

©2014 Bodor. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.