Journal of
eISSN: 2373-633X


Research Article Volume 6 Issue 1
1Department of Internal Medicine, University of Texas Medical Branch, USA
2Sealy Center on Aging, University of Texas Medical Branch, USA
Correspondence: Solly Silwan Chedid, Assistant Professor, Department of Internal Medicine, 301 University Blvd, JSA 4.160, UTMB, Galveston, Texas, TX 77555-0561, USA, Tel 4097728720, Fax 409.747-2369
Received: October 19, 2016 | Published: October 31, 2016
Citation: Washington TM, Chedid SS, Zhang W, et al. Trends of MRI use to evaluate breast cancer in the Texas Medicare population. J Cancer Prev Curr Res. 2016;6(1):388-394. DOI: 10.15406/jcpcr.2016.06.00188
Background: The availability of Magnetic Resonance Imaging (MRI) has increased in the United States over the last decade. The use of MRI to diagnose and stage breast cancer has not been described. We examined the trends and frequency of MRI use in women with newly diagnosed breast cancer in Texas and evaluated the factors associated with receipt of an MRI.
Methods: Medicare-linked data from the Texas Cancer Registry (TCR) were used to identify incident breast cancer cases between Jan. 1, 2003 and Dec. 30, 2007. We included female patients, 65years and older, enrolled in both Medicare Parts A and B for 3months prior to diagnosis and 12months after diagnosis, and not enrolled in an HMO plan during that period. Patients diagnosed at autopsy were excluded. Bivariate and multivariate logistic regression analyses were performed to determine the factors associated with receipt of MRI within 3months before and after diagnosis. A Cochran-Armitage trend test was performed to examine the trends in use of MRI by year. A value of P<0.05 was considered statistically significant.
Results: Of the 18,856 patients meeting inclusion criteria, 1,268 (6.72%) received an MRI. The use of MRI increased significantly in Texas from 2.25% of women diagnosed in 2003 to 13.9% in 2007 (P<0.0001). Bivariate analysis revealed that age, race, number of comorbidities, income, education, and urban/rural location were associated with the receipt of MRI at the time of breast cancer diagnosis. In multivariate analysis, odds of receiving an MRI were lower in women of advanced age (odds ratio OR 0.098; 95% Confidence Interval CI 0.044-0.223), black race (OR 0.66; 95% CI: 0.49-0.88) and the presence of two or more comorbid conditions (OR 0.30; 95% CI; 0.19-0.48). Those with regional disease and a higher level of education were more likely to receive MRI.
Conclusion: Use of MRI in patients with newly diagnosed breast cancer in Texas increased six-fold during the study period, but increases varied across racial groups.
Keywords: breast MRI, breast cancer, MRI, Medicare, Texas
An estimated one in eight women will develop breast cancer in her lifetime.1 Excluding skin cancer, breast cancer is the leading cancer diagnosed in women and the second leading cause of cancer death, accounting for more than one quarter of a million deaths every year.1 The risk of developing breast cancer increases with age. Mammogram imaging to screen for breast cancer was introduced in the 1970s; soon after, the American Cancer Society (ACS) introduced screening guidelines in 19762. Breast ultrasound was introduced to complement screening mammogram but does not replace mammography as the primary screening modality. Breast magnetic resonance imaging (MRI) was introduced in the 1990s and has emerged as an adjunct to the traditional screening methods.
The use of breast MRI has continued to increase as the technology becomes more available; however, the overall clinical benefit of MRI remains unclear.2‒4 Recommendations for screening MRI in addition to mammography have been introduced for women whose risks for breast cancer are increased by inherited cancer syndromes such as hereditary breast and ovarian cancer syndrome (BRCA1 and BRCA2), Li-Fraumeni syndrome, Cowden syndrome, or Bannayan-Riley-Ruvalcaba syndrome.5 With no general recommendations for women at average risk, concerns have been raised that screening breast MRI may be over-utilized in the general population.2 Although some high-risk women may benefit from breast MRI, it may not be as cost-effective in women with low or average risk.
Breast MRI, when combined with mammography and clinical breast examination, has been shown to provide a sensitivity of 99% for the preoperative assessment of the local extent of disease in patients with newly diagnosed breast cancer.6‒8 The overall utilization of MRI for patients with newly diagnosed breast cancer is unclear. The focus of this study is to examine the use of MRI in patients with newly diagnosed breast cancer in the Texas Medicare population and to evaluate the clinical and socioeconomic factors associated with diagnostic (not screening) MRI usage.
Data sources
Medicare-linked data from the Texas Cancer Registry (TCR) were used for the analysis. The TCR–Medicare-linked database is a linkage of two large population-based sources of data, performed under the guidance of the National Cancer Institute (NCI).9 The TCR10 data are collected by the Texas Department of State Health Services while the Medicare claims data are collected by the Centers for Medicare and Medicaid Services.11 This dataset provides detailed information about elderly adults with cancer in Texas. The TCR collects and provides information on participant demographics, cancer prevalence, cancer incidence, tumor characteristics including stage of disease, first course of therapy, and survival. The Medicare claims data include information on hospital stays, physician services, and hospital outpatient visits. The data used in this study include cancer patients diagnosed with primary breast cancer between 2003 and 2007 and their Medicare claims through 2008.
The Internal Review Board (IRB) of the University of Texas Medical Branch (UTMB) approved this study. Because of the nature of the study, written consent was not required.
Study cohort
The initial dataset included all patients diagnosed between Jan. 1, 2003 and Dec. 31, 2007. Patients with primary breast cancer were identified in the database. Men, patients whose diagnoses were made at the time of death or autopsy, and patients who were younger than 65years at diagnosis were excluded. The study was limited to female patients enrolled in both Medicare Parts A and B for 3months prior to diagnosis and 12months after diagnosis but not enrolled in a Health Maintenance Organization (HMO) plan during the same period. Criteria used to establish our study cohort are shown in Figure 1. We examined Medicare claims from outpatient and physician offices (carrier claims) to identify the patients who received MRI within 3months before or after diagnosis. The Healthcare Common Procedure Coding System (HCPCS) and Current Procedural Terminology (CPT) codes were used to detect breast MRI and included the following: CPT codes 77058 and 77509; and HCPCS codes C8903, C8094, C8505, C8906, C8907, and C8908.
Patient socio-demographic characteristics (age, race, residence, and year of diagnosis) and coexisting conditions at the time cancer was diagnosed, along with disease characteristics (stage, surgery type, and tumor size) and whether or not the patient received any chemotherapy were evaluated. United States Census Bureau data for the year 2000 was used to define the percent living below the poverty level and the percent without a high school education, at the census tract level.9 The type of MRI facility (hospital versus freestanding facility) and referring physician specialty (oncology, primary care, surgery, or other) were also obtained from Medicare claims data and examined. For the purpose of this study, primary care provider includes general practitioners, general internists, family practitioners, geriatricians, and obstetricians.
Statistical analysis
The main outcome variable in this study was the receipt of diagnostic MRI. Bivariate chi-squared analysis was performed to identify factors potentially associated with receipt of an MRI. Statistical significance was set at P<0.05. Multivariate analysis was also performed by using the logistic regression method. The model included patient characteristics (age, race, poverty level, education level, residence location, and year of diagnosis) and clinical measures (stage, surgery type, and tumor size). All analyses were performed by using SAS software version 9.2 (SAS Institute, Inc., Cary, NC), and all reported results were based on the two-sided significance level P<0.05.
Of the 37,041 patients who had primary breast cancer between 2003 and 2008, a total of 18,856 met our study criteria. Of these, 1,268 (6.72%) received an MRI within 3months before or after the date of diagnosis. The breast MRI was performed at a mean of 0.469 (Standard Deviation 0.93) months after diagnosis. The mean age of those who received an MRI was 3.1years younger or 72.3years (range 65 to 95years) compared with 75.4years (range 65 to 102years) for those who did not receive a breast MRI (P=0.01).
Table 1 shows the baseline characteristics of patients with the initial diagnosis of breast cancer who had an MRI. Breast MRI was more commonly performed in young, white women. Patients in the lowest income quartile were less likely to receive an MRI than were those in highest income quartile (P<0.0001). Breast MRI was more commonly performed in women with no comorbidities. Women more likely to be offered MRIs tend to be characterized by one or more of the following: residency in a large metropolitan area, higher level of education, invasive lobular carcinoma, regional stage of disease, and tumor size <2cm. Patients who received chemotherapy were more likely to receive an MRI than those who did not receive chemotherapy (P<0.0001. We found no difference in the use of MRI whether patients received adjuvant or neo-adjuvant chemotherapy.
Variable |
Total n=18,856 |
Received MRI n=1,268 (% of total) |
P-value |
Age Group (years) |
|||
65 - 69 |
4,898 |
502 (10.25) |
< .0001 |
70 - 74 |
4,844 |
378 (7.80) |
|
75 - 79 |
4,135 |
224 (5.42) |
|
80 - 84 |
2,931 |
127 (4.33) |
|
85 - 89 |
1,383 |
30 (2.17) |
|
>90 |
665 |
7 (1.05) |
|
Race |
|||
White |
16,452 |
1167 (7.09) |
< .0001 |
Black |
1,622 |
63 (3.88) |
|
Hispanic |
558 |
20 (3.58) |
|
Other |
224 |
18 (8.04) |
|
Comorbidity (n = 18,331) |
|||
0 |
11,562 |
942 (8.15) |
< .0001 |
1 |
4,243 |
226 (5.33) |
|
2 |
1,473 |
72 (4.89) |
|
>2 |
1,053 |
23 (2.18) |
|
Poverty Level (n = 17,511) |
|||
1st Quartile ($$$) |
4,381 |
440 (10.04) |
< .0001 |
2nd Quartile |
4,376 |
309 (7.06) |
|
3rd Quartile |
4,389 |
237 (5.40) |
|
4th Quartile ($) |
4,365 |
159 (3.64) |
|
Education Level (n = 17,511) |
|||
1st Quartile (highest) |
4,379 |
448 (10.23) |
< .0001 |
2nd Quartile |
4,387 |
301 (6.86) |
|
3rd Quartile |
4,382 |
225 (5.13) |
|
4th Quartile (lowest) |
4,363 |
171 (3.92) |
|
Urban/Rural (n = 18,852) |
|||
Large Metro |
9,420 |
808 (8.58) |
< .0001 |
Metro |
5,115 |
215 (4.20) |
|
Urban |
1,272 |
63 (4.95) |
|
Less Urban |
2,644 |
155 (5.86) |
|
Rural |
401 |
26 (6.48) |
|
Stage |
|||
0/In situ |
2,846 |
179 (6.29) |
0.0337 |
Localized |
9,889 |
672 (6.80) |
|
Regional |
4,325 |
320 (7.40) |
|
Distant |
614 |
39 (6.35) |
|
Unknown |
1,182 |
58 (4.91) |
|
Surgery Type |
|||
Breast Conservation |
10,933 |
757 (6.92) |
< 0.1605 |
Mastectomy |
4,831 |
327 (6.77) |
|
None/Other |
3,092 |
184 (5.95) |
|
Tumor Size |
|||
<=2 |
9,101 |
652 (7.16) |
0.0468 |
> 2 |
6,672 |
431 (6.46) |
|
Unknown |
3,083 |
185 (6.00) |
|
Year |
|||
2003 |
4,049 |
91 (2.25) |
< .0001 |
2004 |
3,823 |
136 (3.56) |
|
2005 |
3,730 |
183 (4.91) |
|
2006 |
3,678 |
361 (9.82) |
|
2007 |
3,576 |
497 (13.90) |
|
Histology |
|||
Invasive ductal carcinoma |
14,333 |
873 (6.09) |
<.0001 |
Invasive lobular carcinoma |
1,503 |
181 (12.04) |
|
Non infiltration carcinoma |
613 |
40 (6.53) |
|
Metaplasia |
73 |
4 (5.48) |
|
Other |
2,334 |
170 (7.28) |
|
*Chemotherapy (n=15,764) |
|||
Yes |
3,170 |
301 (9.50) |
<.0001 |
No |
12,594 |
783 (6.22) |
|
Table 1 Subject demographics and percent of patients receiving breast magnetic resonance imaging (MRI) within 3 months before or after initial breast cancer diagnosis
*Chemotherapy: chemotherapy was investigated among those 15,764 patients who had surgery within 4 months after diagnosis of breast cancer.
Table 2 shows the multivariable analyses for the receipt of MRI at the time of initial breast cancer diagnosis. After adjusting for age group, race, comorbidities, poverty level, education level, urban/rural location, stage of disease, surgery type, tumor size, year of diagnosis, and histology, black women had lower odds of receiving an MRI at the time of breast cancer diagnosis compared with non-Hispanic whites (odds ratio OR 0.663; 95% Confidence Interval CI 0.495-0.887). Having just a single comorbidity was associated with a 29% reduction in the receipt of MRI when compared with patients with no comorbidities. Not surprisingly, when compared with patients with no comorbidities, patients with two or more comorbidities had 0.30 odds (95% CI; 0.19-0.48) of receiving an MRI (P<0.0001). The statistical association between poverty levels and MRI receipt seen in the bivariate analysis did not persist after adjusting for other covariates in the multivariate analysis.
Variable |
Odds of MRI (95% Confidence Interval) |
Pr > ChiSq |
Age Group (years) |
||
65 - 69 |
1 |
< 0.0001 |
70 - 74 |
0.780 (0.669 – 0.909) |
|
75 - 79 |
0.508 (0.424 – 0.609) |
|
80 - 84 |
0.405 (0.327 – 0.503) |
|
85 - 89 |
0.209 (0.141 – 0.310) |
|
>90 |
0.097 (0.043 – 0.220) |
|
Race |
||
White |
1 |
0.0525 |
Black |
0.663 (0.495 – 0.887) |
|
Hispanic |
0.962 (0.585 – 1.583) |
|
Other |
0.921 (0.539 – 1.575) |
|
Comorbidity |
||
0 |
1 |
< 0.0001 |
1 |
0.710 (0.604 – 0.836) |
|
2 |
0.725 (0.555 – 0.948) |
|
>2 |
0.303 (0.192 – 0.479) |
|
Poverty Level |
||
1st Quartile ($$$) |
1 |
0.6152 |
2nd Quartile |
1.051 (0.866 – 1.274) |
|
3rd Quartile |
1.020 (0.796 – 1.306) |
|
4th Quartile ($) |
0.886 (0.651 – 1.207) |
|
Education Level |
||
1st Quartile (highest) |
1 |
0.0001 |
2nd Quartile |
0.733 (0.608 – 0.884) |
|
3rd Quartile |
0.597 (0.466 – 0.764) |
|
4th Quartile (lowest) |
0.547 (0.406 – 0.737) |
|
Urban/Rural |
||
Large Metro |
1 |
< .0001 |
Metro |
0.470 (0.392 – 0.565) |
|
Urban |
0.560 (0.407 – 0.772) |
|
Less Urban |
0.869 (0.695 – 1.085) |
|
Rural |
0.980 (0.614 – 1.564) |
|
Stage |
||
0/In situ |
1 |
0.017 |
Localized |
1.131 (0.921 – 1.389) |
|
Regional |
1.280 (1.016 – 1.613) |
|
Distant |
1.151 (0.761 – 1.742) |
|
Unknown |
0.595 (0.373 – 0.951) |
|
Surgery Type |
||
Breast Conservation |
1 |
0.9674 |
Mastectomy |
1.002 (0.861 – 1.167) |
|
None/Others |
0.975 (0.793 – 1.199) |
|
Tumor Size |
||
<=2 |
1 |
0.3239 |
>2 |
0.911 (0.787 – 1.054) |
|
Unknown |
1.054 (0.842 – 1.319) |
|
Year Diagnosed |
||
2003 |
1 |
< 0.0001 |
2004 |
1.620 (1.219 – 2.153) |
|
2005 |
2.424 (1.852 – 3.173) |
|
2006 |
4.749 (3.705 – 6.089) |
|
2007 |
7.541 (5.917 – 9.610) |
|
Histology |
||
Invasive ductal carcinoma |
1 |
< 0.0001 |
Invasive lobular carcinoma |
2.087 (1.725 – 2.525) |
|
Non infiltration carcinoma |
1.388 (0.964 – 2.000) |
|
Metaplasia |
1.019 (0.308 – 3.364) |
|
Other |
1.248 (1.032 – 1.510) |
|
Table 2 Multivariate analysis of the odds of receiving magnetic resonance imaging (MRI) at the time breast cancer is diagnosed
As patient education levels increased, so did the odds of receiving an MRI. Patients in the 4th quartile (lowest) had 0.55 (5% CI; 0.41- 0.77) lower odds of receiving an MRI than those in the 1st quartile (highest). This disparity was seen also in patients living in less populated areas compared with those living in large metropolitan areas. When compared with patients who had in situ stage of disease, those with regional stage disease had higher odds 1.28 (95% CI; 1.02-1.61) for receipt of MRI.
Figure 2 shows the use of MRI as a proportion of total numbers of cases diagnosed by each year of the study. The proportion rose each year. After adjusting for other covariates, patients diagnosed in 2007 were 7.6times more likely to have an MRI compared with those diagnosed in 2003 (Table 2).
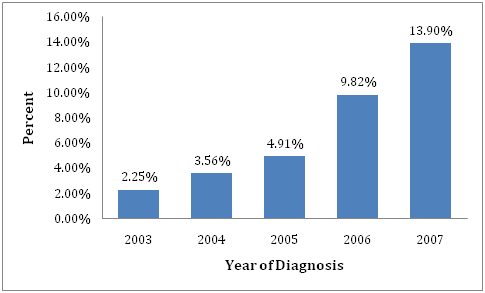
Figure 2 Percent of patients receiving Magnetic Resonance Imaging by year of diagnosis of breast cancer (2003-2007).
Histology also influenced MRI receipt. Patients with invasive lobular carcinoma had greater odds of receiving an MRI that those with invasive ductal carcinoma. In the multivariate analysis, patients with invasive lobular carcinoma were more than twice as likely to receive an MRI than were patients with invasive ductal carcinoma (P<0.0001). Finally, we looked at the physicians who ordered an MRI at the time of breast cancer diagnosis. In our study, we found that primary care providers ordered 38% (n=465) of the MRIs, while surgical specialty physicians ordered 33% (n=398) and oncology specialties ordered only 6% (n=70).
The National Comprehensive Cancer Network (NCCN) has provided recommendations for the use of a dedicated breast MRI in the initial workup of breast cancer as either a category IIA or IIB, which means that the NCCN recognizes the limitation of the breast MRI and the lack of strong evidence for the benefit of MRI in the evaluation of breast cancer.12
In our study, we found that women diagnosed with breast cancer who had higher education, lived in large metropolitan areas, and had a higher income were more likely than other women to have an MRI at the time of diagnosis. Ethnicity of women who received an MRI at the time of diagnosis was also a factor, with whites more likely to receive an MRI and blacks 33% less likely. Patients with more than two comorbidities were 70% less likely to get an MRI than those with no comorbidities.
Our finding that women diagnosed with breast cancer in Texas in later years were more likely to receive an MRI during the diagnostic period is consistent with a recent study using the Surveillance Epidemiology and End Results (SEER) Medicare data.13 Overall, use of MRI was found to increase significantly from 3.9% of women diagnosed in 2003 to 10.1% of women diagnosed in 200514. Our study included only Texas residents but confirmed the finding of the SEER study, showing that breast MRI utilization differed significantly by region. Breast MRI use was as high as 18% in New Mexico compared with 0.1% in Hawaii for women with newly diagnosed breast cancer over the age of 65years14, whereas 7% of newly diagnosed Texas women received a diagnostic MRI. As in our study, the SEER-Medicare multivariate analysis showed that young, white women with higher socioeconomic status were more likely to receive an MRI during diagnosis when compared with others.13
These findings in Texas, as with the national SEER finding, are important because although black patients have a lower incidence of breast cancer, they have higher breast cancer mortality than their white counterparts.14,15 Likewise, lower socioeconomic status has been associated with increased mortality.14‒16 Because the efficacy of diagnostic MRI on overall breast cancer outcomes is controversia,17 whether not having an MRI contributes to the differences in disease mortality remains unknown.
Invasive lobular breast cancer is the second most common type of breast cancer.18 As invasive lobular carcinoma of the breast has a higher rate of multicentric/multifocal and contralateral disease than invasive ductal carcinoma, lobular carcinoma has been associated with increased MRI use in previous studies.19‒20 Our findings were similar, with patients having invasive lobular carcinoma more than twice as likely to receive an MRI than those with invasive ductal carcinoma. The increased use of diagnostic MRI for lobular histology is appropriate22‒25 and is supported by NCCN guidelines, and the American Society of Breast Surgeons (https://www.breastsurgeons.org/statements/index.php).
Another indication for diagnostic MRI is to assist with neoadjuvant versus adjuvant chemotherapy recommendations.26 Neoadjuvant chemotherapy can be used to decrease the size of large tumors in patients who want breast conservation therapy, but may not be good candidates for breast conservation initially. Also, patients who are deemed unresectable may become operable candidates after neo-chemotherapy. Most breast cancer histology will have some response to chemotherapy and 9-26% of the patients who get neoadjuvant chemotherapy will achieve a complete pathologic response.27,28 In our study, patients who received chemotherapy were more likely to have an MRI at diagnosis. However, there was no difference in the utilization of MRI by whether patients received adjuvant or neoadjuvant chemotherapy. In a previous study, the most common reason to order a breast MRI was for breast cancer screening, which is done predominantly by primary care providers.29 In our study, we found that for diagnostic MRIs, the primary care physicians ordered 38% of MRIs, surgeons ordered 33%, and oncologists only ordered about 6%.
The purpose of our study was to examine the factors associated with utilization of diagnostic MRI. Diagnostic MRI use has been reported to possibly influence treatment decisions.26,27,30 However, since administrative datasets do not contain information to establish risk or intent of treatment, we could not study how diagnostic MRI usage influenced the surgery. We did not assess the timing of each modality studied, but rather whether or not each (surgery or chemotherapy) occurred within the same 3-month window as the diagnostic MRI. In this descriptive study, we did observe that patients receiving mastectomy were just as likely to get an MRI as those who received breast conservation.
Our study design did not allow us to assess pre-operative use of MRI. Although, MRI has been shown to detect more multicentric/multifocal disease than conventional imaging6,31‒33 and to aid in the identification of contralateral breast lesions, breast MRI is not reliable at distinguishing benign from malignant lesions.28,34‒36 Only the biopsy of lesions visualized by MRI is required to determine the histology. However, contralateral breast lesions appear to occur in 2% to 10% of patients diagnosed with unilateral breast cancer by traditional mammography 6, 33, Turnbull et al.,30 reported that 13 (2%) additional patients who were found to have contralateral lesions with MRI who were missed by use of mammogram alone at the time of diagnosis17 in the COMICE trial, a multicenter randomized control trial comparing diagnostic MRI versus no MRI prior to partial mastectomy. Excessive use of intraoperative MRI could lead to unnecessary surgical procedures, such as biopsy of false positive lesions visualized by MRI only, and total mastectomy instead of partial mastectomy for treatment. Magnetic resonance imaging has been found to be very useful in younger patients because their breast tissue tends to be more dense. Although our study shows a trend towards younger patients getting MRI in the perioperative (diagnostic) setting, our study was limited to patients over age 65years.37‒43
The collection of cancer incidence data used in this study was supported by the Texas Department of State Health Services (DSHS) and Cancer Prevention Research Institute of Texas (CPRIT), as part of the statewide cancer reporting program, and the National Program of Cancer Registries Cooperative Agreement #5U58/DP000824-05 of the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the DSHS, CPRIT, or CDC.
Authors declare there are no conflicts of interest.

©2016 Washington, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.