Journal of
eISSN: 2373-633X


Research Article Volume 14 Issue 4
1Department of Biological Science, Faculty of Science, Confluence University of Science and Technology, Nigeria
2Department of Biochemistry, Faculty of Science, Federal University Lokoja, Nigeria
3Department of Science Laboratory Technology, Federal Polytechnic Ayede, Nigeria
4Department of Biochemistry, Faculty of Natural Science, Prince Abubakar Audu University, Nigeria
Correspondence: Victor Duniya Sheneni, Department of Biochemistry, Faculty of Science, Federal University Lokoja, Nigeria, Tel +234-8033519009, Fax +234-8088769425
Received: July 26, 2023 | Published: August 17, 2023
Citation: Momoh IS, Sheneni VD, Akomolafe AP, et al. Treatment challenges associated with breast cancer and chemotherapeutic drug resistance. J Cancer Prev Curr Res. 2023;14(4):77-81. DOI: 10.15406/jcpcr.2023.14.00525
The most frequent form of cancer in women worldwide and the main factor in mortality from cancer, breast cancer is a common malignancy. In 2012, there were 521,900 cancer deaths and an estimated 1.7 million new cases of cancer (or 25% of all cancer kinds). Women are at risk for breast cancer for a variety of reasons, but breast density, genetic predisposition, age, and estrogen dysregulation stand out. A heterogeneous illness like breast cancer is brought on by shared ecological and hereditary factors. Development of novel molecularly targeted therapeutics is facilitated by a thorough understanding of the genesis of breast cancer. Numerous variables, including tumor size, lymph node involvement, and the presence of estrogen, progesterone, and HER2 receptors, determine the therapeutic efficacy of chemotherapy. One of the main issues with cancer chemotherapy, particularly in the case of breast tumors, is drug resistance. In order to achieve this, a number of active trials are investigating brand-new medication combinations that aim to block important signaling pathways that contribute to the development of disease. These therapeutic advancements may help individuals with breast cancer overcome their treatment resistance. Additionally, finding additional biomarkers and possible drug targets may help to create new chemotherapeutic combinations that eventually increase the effectiveness of these combination therapies.
Keywords: chemotherapy, drug resistance, risk factors, breast cancer
Breast cancer (BC) is the most often diagnosed malignancy in women and the primary cause of cancer deaths worldwide, with an estimated 1.7 million new cases and 521,900 deaths in 2012 (Figure 1).1
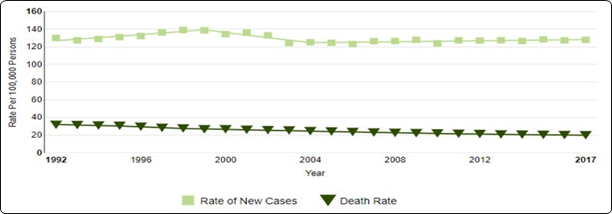
Figure 1 Number of New Cases and Deaths per 100,000.
Adapted from https://seer.cancer.gov/statfacts/html/breast.html
It affects about 12% of women worldwide and is regarded as the second most frequently occurring invasive cancers. Breast cancer was deemed to be the most common female malignancy in 2012 when it was found to comprise 25.2% of all malignancies, with prevalence rates in developed and underdeveloped nations ranging from 89.7 per 100,000 to 19.4 per 100,000. However, during the same year, lung cancer was the cause of 12.8% of all cancer-related deaths in women. Since women are more likely to get breast cancer than men, 18.2% of all cancer-related deaths were caused by cancer generally, but breast cancer only accounted for 6% of all cancer-related deaths in both men and women. Early cancer identification and the variety of risk factors for breast cancer have a role in the variations in cancer frequency around the world. In the United States, breast cancer is regarded as the most prevalent and second-most common cause of fatalities among female cancer patients.1-9 A study estimates that there will be 246,660 new cases of female breast cancer in 2016, making up 14.6% of all new cases. Of these, 40,450 women are anticipated to pass away from this cancer. Around 12.3% of women can expect to receive a breast cancer diagnosis at some point in their lives. Breast cancer is probably more common in women than in males; in 2015, about 2300 men received a diagnosis and 440 died as a result of the disease. Between the ages of 60 and 84, white women experience a noticeably higher incidence of breast cancer than black women. But before the age of 45, breast cancer is more likely to affect black women. When compared to other racial and ethnic groups, breast cancer-related fatalities are more common among non-Hispanic black and white women. Except for Alaska Natives and American Indians, all ethnic groups saw the same rate of overall breast cancer incidence from 2004 to 2012 (Figure 2).9,10 However, these groups experienced a yearly decline in the number of new cases.
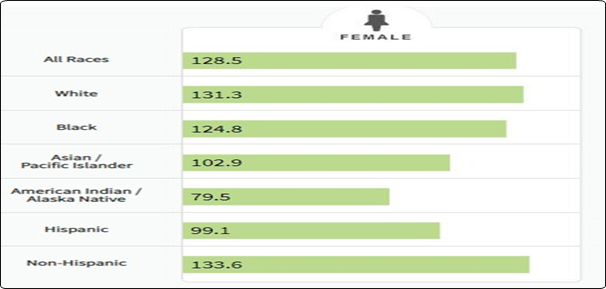
Figure 2 Number of New Cases per 100,000 Persons by Race/Ethnicity.
Adapted from https://seer.cancer.gov/statfacts/html/breast.html
Although the primary cause of this decline in instances is still unknown, it may be due to recent advancements in malignancy therapy and an increase in survival rates (Figure 3).
A crucial step in the diagnosis of breast cancer is risk assessment of the patient. The suggestion for tests as well as patient preferences may be influenced by the assessed risk of a person. Breast cancer risk factors include genetic predisposition, aging, and estrogen dysregulation. Breast density, on the other hand, is regarded as a substantial risk factor as well. With the exception of a few inherited disorders like BRCA, the risk factor is minimal but may have an impact on other factors. Breast cancer is mostly a diverse illness brought on by inherited and environmental causes. Age, obesity, alcohol use, and hormone dysregulation are all risk factors for breast cancer, according to epidemiological research, but family history is the most significant one. According to reports, 20% of all breast cancers have a familial history. At age 40, 50, and 60 in the United States, the probability of developing a malignant breast tumor is correspondingly 1.5%, 2.3%, and 3.5%.11-18 In women, the chance of getting breast cancer is influenced by several hormone levels. Females who are pre- or post-menopausal may be at an increased risk due to high levels of endogenous estrogen.14 Other common risk factors include breast tissue radiotherapy, obesity, drinking alcohol, giving birth later in life or not at all, taking multiple hormones (estrogen and progesterone) to delay menopause symptoms, the appearance of dense breast tissue on mammograms, family history of breast cancer (first-degree relatives), personal history of having benign breast growths or invasive breast cancer, ductal carcinoma in situ (DCIS), or lobular c.
Molecular classification of breast cancer
Breast cancer is diverse and separated into three different sub types including luminal, human epidermal growth factor receptor 2+ (HER2+), and basal breast like breast tumors based on molecular or gene expression profiling. According to therapy response, risk of disease progression, and organ choice for tumor metastasis, each kind behaves differently. The majority of luminal tumors are estrogen and progesterone receptor positive (ER/PR+), and they respond well to hormone-based therapies. However, HER2+ tumors have overexpressed the oncogene ERBB2, which can be effectively controlled by utilizing various anti-HER2 therapy strategies. Contrarily, basal-like cancers lack HER2 and hormone receptors; as a result, the majority of these tumors fall under the category of triple-negative breast cancer (TNBC). Unfortunately, these tumors do not yet respond well to available traditional chemotherapeutic drugs (less than 20%), and there are no such targeted molecular therapies for them. In several investigations, the origin of inter-tumor heterogeneity in breast malignancies was proposed. Accordingly, basal-like malignancies derive from differentiated stem-like cells, whereas luminal lineages are dedicated progenitors of luminal and HER2+-based tumors. However, gene expression patterns and experimental data imply that the following genetic and epigenetic variables may cause luminal progenitors to operate as basal-like cancers' precursor.19–25
The heterogeneity of breast cancer
Breast cancer is a varied malignancy rather than a straightforward disease, as was established by morphological findings prior to the development of contemporary molecular profiling techniques. It was categorized based on a number of variables, including the tumor grade, lymph node status, and the presence of recognizable markers such the estrogen receptor (ER) and, more recently, the human epidermal growth factor receptor 2 (HER2). Through the use of DNA microarray data analysis and molecular profiling, the heterogeneity of breast cancer was further demonstrated. The presentation was based on the genetic and immunohistochemical expression of ER, PR, and HER2 breast cancer, and it was further split into five clinical sub groups: luminal A, luminal B, HER2, basal, and normal breast cancer.
Targeted therapies against breast cancer
A thorough understanding of the molecular causes of breast cancer paves the way for the discovery of numerous molecular targets and the creation of innovative treatments. The tyrosine kinase inhibitors (TKIs) target HER1, HER2, HER3, IGF receptor (IGFR) and FGF receptor (FGFR) as well as intracellular pathway inhibitors (PI3K, ERK, AKT, and mTOR), angiogenesis inhibitors, and other substances that obstruct DNA repair mechanisms. Some of these inhibitors, such as lapatinib, transtuzumab, and anti-HER2 medicines, demonstrated outstanding activity and are used successfully in the treatment of breast cancer. A monoclonal antibody called transtuzumab has been shown to be the most successful treatment for women with HER2+ breast cancers.26-32 Bevacizumab, a monoclonal antibody that targets vascular endothelial growth factor (VEGF), is more effective than other potential drugs like lapatinib, a dual inhibitor of EFGR; HER1 and HER2.
The estrogen paradox
Targeting ER itself is important for the treatment of ER+ breast tumors. Treatment options include employing specific anti-estrogen tamoxifen or reducing the amount of available ligands (estrogen) for the receptor. These endocrine medications are frequently used to treat early, metastatic, and recurring breast cancer due to their demonstrated efficacy. Breast cancer is known to be an estrogen-based malignancy when risk factors are taken into account. Breast cancer, however, does not manifest as a disease until the body's production of estrogen has been reduced in women. Nearly 10 years following menopause, at age 62, is when this condition is most common. When estrogen levels are at their maximum, less than 5% of all breast cancers develop in those under the age of 50. To explain this absurdity, several hypotheses can be put forth.33–35 Every proliferative cycle increases the likelihood of genetic and epigenetic mistakes (overexpression of oncogenes and methylation of tumor suppressor genes), which each menstrual cycle has a chance of introducing into compartments of mammary epithelial cells. Breast cancer risk is also increased by other DNA repair system flaws (such as BRCA or p53 mutations).34
Chemotherapy
Chemotherapy's benefits are dependent on a number of variables, including the tumor's size, lymph node involvement, the existence of hormone receptors (estrogen and progesterone), and the overexpression of HER2 in cancer cells. According to research, TNBCs and HER2+ breast cancers respond to chemotherapy more readily than HR+ breast tumors. A few gene expression panels (PAM 50, Oncotype DX, and Mammaprint) are the assays to assess the likelihood of recurrence in HR+, HER2-breast cancers at an early stage. These variables may help determine both the candidates who might benefit from chemotherapy and those who could safely avoid it. Oncotype DX 21-Gene Recurrence Score is popular in the United States. A high DX 21-gene score indicates who should probably receive adjuvant chemotherapy and hormone treatment, whereas a low score indicates who should probably stay away.
The patient's age and the size of the tumor have absolutely no bearing on the scores that follow. Multiple studies have found that pharmacological combinations, as opposed to single therapies, are more effective at treating breast cancers in their early stages. Adjuvants and neoadjuvant therapy often lasts for three to six months depending on the chemistry of the medications. The combination therapy is very effective when the dose and drug cycles are finished on schedule if interruptions or severe delays could be avoided. Additionally, broad guidelines have been established to help oncologists decide when and what kind of therapy to administer. The characteristics of the malignancy (HR, HER2 status, stage of the cancer, grade, and lymphovascular invasions) and patient-related considerations (possible benefits, potential toxicity, life expectancy, age, and patient preference) should be considered when deciding whether to administer chemotherapy. Numerous studies suggested that polychemotherapy was preferable to monochemotherapy. Anthracycline use has entered routine medical practice, but there is a higher risk of heart toxicity involved; in contrast, taxanes' long-lasting advantages should outweigh their risk of long-term neuropathy.36–41
Chemotherapeutic drug resistance
One of the biggest issues with cancer chemotherapy is drug resistance, particularly when treating breast tumors. The increased rate of motility is a sign that chemotherapy has not yet been able to cure the illness. Since the rise of medication resistance in the context of breast cancer, the difficulties with chemotherapy have increased. Resistance typically falls into one of two categories: either poor drug delivery to cancer cells, which results in poor absorption of the drug, or higher excretion, which results in lower levels of drug in the bloodstream and, ultimately, decreased drug availability to the tumor tissue. The second factor is the epigenetic modifications that affect drug sensitivity. These epigenetic modifications include histone deacetylation, DNA methylation, and nucleosome rearrangement. The tumor suppressor gene is silenced as a result of these epigenetic alterations. Other modifications disrupt apoptotic and growth regulatory mechanisms, reactivate oncogenes, and activate oncogenes. These alterations can, however, occasionally be reversed by adding certain inhibitors, for example.
Drug resistance has been linked to a variety of mechanisms, including changes in drug transport, pharmacological targets, drug metabolism, drug-detoxifying systems, improved DNA repair, and deregulated apoptotic pathways. Additionally, when transplanted into animal models, certain monolayer cells that are drug-sensitive in cell culture develop resistance.42–52 This is a sign that extracellular matrix, tumor shape, or other environmental variables are contributing to treatment resistance. Drug resistance may be influenced by cells developing in three-dimensional (3D) shapes in cell culture that mimic in vivo geometry.43,52,53 In cell culture, cancer cells can easily develop resistance to a single agent or a class of agents with comparable mechanisms of action by changing the way that drugs cause their DNA to be damaged or by enhancing the DNA repair process.
A cell may exhibit cross-resistance to various mechanistically or structurally distinct classes of medicines after developing resistance to a single agent. Multidrug resistance is the term used to describe this phenomenon. The development of ATP dependent efflux pumps, which are members of the ATP-binding cassette (ABC) transporter family and share structural and sequence similarity, is the primary cause of resistance to some natural or hydrophobic medicines, often known as classical multidrug resistance. The medications that are impacted by multidrug resistance include anthracyclines, doxorubicin and daunorubicin, vinca alkaloids, vinblastine and vincristine, therapies for stabilizing microtubules, and Paclitaxel, an inhibitor of RNA-transcription actinomycin-D. One of the key mechanisms of multidrug resistance is increased expression of the protein P-glycoprotein.54-57 Pgp is a membrane-associated glycoprotein with a maximum molecular weight of 170 kDa that can extrude doxorubicin and other cytotoxic substances from the cytoplasm to the exterior of the cell, lowering the concentration of the medication inside the cel.42
The development of our knowledge of the genesis of breast cancer over the past century has benefited the employment of various techniques. Each approach is based on one of the numerous facets of this illness, but no one approach is effective for treating all forms of cancer. By the time we switched from the approach of completely eliminating malignancies to causing long-term cell inactivity. This strategy may be comparable to therapy because it allows the patient to live out their natural lifespan without the cancer worsening and prevents the cancer from waking up. The breast cancer needs a number of alterations to start and spread within the body. Strong hormone drive, diverse genetic and epigenetic alterations, a weakened immune system, and a persistent inflammatory environment are some of these changes. Damage to the intracellular regulatory system, a considerable drop in apoptosis, and a tolerant microenvironment round out the list of four. The volatility that came before is magnified by inherited variables. The patient's reaction to a particular treatment and many risk variables determine the interpatient heterogeneity.
Intrapatient heterogeneity and clonal evolution are caused by a persistent evolutionary stress caused by a variety of host dependent variables and utilized treatments. Heterogeneity across and among patients is seen as one of the main obstacles to treating breast cancer. Genomic tools made a significant contribution to the selection of a particular course of treatment for each patient, thereby lowering the morbidity and mortality rates associated with medical care. However, to improve our estimation of the benefits from existing medicines, the development of biomarkers and diagnostic tools is still required. Chemotherapy for the patient aids in cell cycle arrest or DNA damage induction. The latest regimens, however, are reportedly less harmful to normal cells and more effective than the previous ones. Chemotherapy research appears to have reached a plateau over the previous ten years due to a lack of significant or unique developments. Unfortunately, one of the main obstacles to breast cancer treatments now in use is medication resistance. Any anticancer medicine's therapeutic effectiveness is reduced by the drug resistance mechanism, particularly in patients who have already tried every possible treatment option.
Though it is envisaged that gene profiling may help in the selection of individuals who will benefit from specific treatments, the development of drug resistance is a major barrier. To this purpose, a number of ongoing trials are investigating novel, potent combinations that aim to block well-known cell signaling pathways that are important in disease progression. Any breast cancer patient will benefit from these advancements in treatment as they continue to combat drug resistance and disease progression. The development of new chemotherapy combinations that will eventually increase the efficacy of current combination therapies may be aided by the identification of additional biomarkers and possible therapeutic targets. For the therapeutic treatment of breast cancer, more study on these molecular targets can be suggested.
None.
Authors declare that there is no conflict of interest.

©2023 Momoh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.