Journal of
eISSN: 2373-633X


Review Article Volume 3 Issue 2
University of Northampton, UK
Correspondence: Jess Aimee White, University of Northampton, 31 Windermere Way, Northampton, NN3 6PA, Tel 07932697812;
Received: July 28, 2015 | Published: October 19, 2015
Citation: White JA. The regulation and expression of hepcidin in carcinogenesis as a result of iron overload disorders: an extended literature review. J Cancer Prev Curr Res. 2015;3(2):252-263. DOI: 10.15406/jcpcr.2015.03.00077
Iron is an essential nutrient with limited bioavailability, which allows for the cell cycle progression of G1/S phases. When present in excess, iron poses a threat to cells and tissues, and therefore iron homeostasis has to be tightly controlled. Iron's toxicity is largely based on its ability to catalyse the generation of radicals, which attack and damage cellular macromolecules and promote cell death and tissue injury. This is lucidly illustrated in diseases of iron overload, such as hereditary hemochromatosis or transfusional siderosis, where excessive iron accumulation results in tissue damage and organ failure. Pathological iron accumulation in the liver has also been linked to the development of hepatocellular cancer. The regulation of iron however, is maintained by both hepcidin and ferroportin. Hepcidin is a peptide hormone secreted mainly by hepatocytes; regulating iron homeostasis by binding to the transporter protein ferroportin and inducing its internalisation and degradation. It has been suggested that oxidative damage from reactive oxygen species (ROS) will only occur at particular concentrations of hepcidin, in both deficient and excessive states. As hepcidin has a significant role in iron homeostasis, both states are shown to prompt cell proliferation and reduce transitional progression through the cell cycle. As well as its role in iron metabolism, hepcidin has significant functional roles in several signalling pathways, such as the JAK-STAT pathway and the BMP pathway. Clinical evidence from the past 20years, has also shown a suggested therapeutic use, which targets hepcidin regulation in both anaemic and iron-overload cancer patients. Despite thorough research, there is little evidence to provide a definite use on clinical terms.
As part of the research project, an extended literature review was undertaken to distinguish the relationship between hepcidin, the iron regulatory protein, and its role in the progression of carcinogenesis, based on regulation and expression in iron abundant cells. The review also aims to critically assess hepcidin’s role in iron metabolism and the link between iron overload/deficiency disorders, such as thalassemia, with the normal biological functioning of the immune system. Finally, the project aims to highlight the therapeutic uses of hepcidin in both anaemic and iron overload cancer states, with regards clinical evidence.
Biological importance of iron
Maintaining iron concentration: Iron is the necessary molecule for cellular metabolism and is an imperative nonorganic substance, that plays a major role in oxygen transport, short-term oxygen storage,1 electron transfer,2 DNA synthesis and cell cycle phase transitioning.3 CDK mRNA levels, for example, can be up- or down-regulated by these cell cycle control factors and triggered protein expression. Iron’s physiological importance, however, is primarily determined by its oxidative state, where it has the ability to change between its ferrous (Fe2+) and ferric (Fe3+) form.4 Thus, potentially making it a beneficial component for haemoglobin, cytochromes and several other enzymes. As well as this, iron’s chemical state is important for the understanding of inorganic iron transport that is independent of transferring.5
Iron’s capability to accept and donate electrons also characterises this molecule as a toxic metal and is able to arise via the Fenton reaction, producing free radicals from converted hydrogen peroxide (H202). Thus, can cause significant damage to any proteins, DNA and fatty acids that occur within the given cell.6 The maintenance of iron concentration is, therefore, imperative for normal biological functioning and biological systems have derived several transport and storage processes that work to achieve this homeostasis.
Distribution of iron: The normal level of body iron predominantly occurs between 60-170μg/dL.7 Where approximately, 65 % is integrated into erythroid cells, 30% is stored within liver cell lines and bone marrow as ferritin and the remaining 5% is circulated to both myoglobin and transferring.8 Some circulating serum iron, however, produced by the liver must be either stored or used, to avoid the dysregulation of the bodies iron concentration.9
Absorption of iron: Iron absorption primarily occurs in the duodenum and upper jejunum10 and is based on a feedback mechanism that either increases or decreases iron absorption, in response to a deficient or overload state. Iron predominantly acquired from our diet is absorbed in one of two forms: as haem iron or non-haem iron. Haem iron absorption is thought to be highly efficient due to the proteolytic digestion of haemoglobin, which often results in the haem being released ready for absorption.5 Non-haem, however, has a significantly lower efficiency in comparison. Ferric iron must first be altered to its ferrous form before absorption can occur5 and is mainly transported by the divalent metal transporter 1 (DMT1) (Figure 1). During a transferrin cycle, DMT1 transports Fe2+ iron into the duodenum and upper jejunum and out of the endosome.11 It is thought that post-natal mice lacking DMT1 often show increased levels of iron depletion and, in turn, are characterised with either iron deficiency anaemia or overload.12
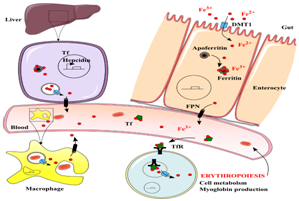
Figure 1 Iron uptake, transport, delivery and recycling in the human body.123
Consequently, intracellular iron must either be stored in ferritin or exported via the iron transporter protein known as ferroportin (FPN1). As mentioned previously, iron when in its ferrous state, must be oxidised to its ferric state, before it can be loaded on to the glycoprotein apotransferrin and exported.13 McKie et al.14 similarly, investigated this process, based on the overexpression of ferroportin in the presence of DMT1 and found that this significantly increased the efflux of iron, due to the lack of its mediator hepcidin.
The mechanism in which ferroportin plays a role in the absorption of iron is highly regulated by the hormone hepcidin, which is triggered for release when there is an increase in iron levels. The interaction between ferroportin and hepcidin can be demonstrated by studies focusing on cell cultures, where hepcidin is added to ferroportin expressing cells. This in turn, shows the internalisation and degradation of ferroportin itself.15 The mechanistic action between these two proteins, therefore, controls the level of iron entering the serum and prevents the build-up of excess iron.15
Finally, with regards to absorbed ferric iron, this is predominantly bound to apotransferrin. Transferrin (Tf) is a major binding protein found in the serum that transports ferric iron from the intestine and liver parenchymal cells to proliferating cells within the body.13 Transferrin, however, can only facilitate the transport of iron into the cells which express complementary transferrin receptors and similarly, prevents the production of reactive oxygen species (ROS).15
Iron homeostasis regulation
Cellular iron uptake: Iron uptake is primarily dependent on transferrin receptor (TfR) expression. A transferrin receptor is a carrier protein, which is needed for the transportation and uptake of iron into the cell and is regulated in response to intracellular changes in the concentration of iron. Iron is transported and taken up by the internalisation of the transferrin-iron complex through receptor-mediated endocytosis.16 Low levels of iron are thought to promote an increase in TfR expression, in order to increase the uptake of iron into the target cell. Thus, maintaining cellular iron homeostasis.17 TfR expression is also controlled by the iron regulatory element binding protein (IRE-BP or IRP) [16]. When iron is not bound, this protein has the ability to bind to a hairpin-like structure (IRE) and inhibit the degradation of mRNA for IRE; which, in turn, also regulates the cellular iron concentration.17
There are two forms of the transferrin receptor. TfR1, which has a ubiquitous expression and TfR2, which is only found to be expressed in the liver.18 The binding of transferrin to the TfR1 receptors causes the endocytosis of the transferrin-iron complex. Aisen P19 has suggested that the endosome is likely to be acidified, due to the establishment of a proton pump which, promotes a conformational change in transferrin. Thus, releases iron, ready to be oxidised to its ferrous state; iron can then be transported by DMT1. When no longer bound, the apotransferrin (apoTf) is moved back to the cell surface where it will undergo another cycle of iron transport and uptake.
While TfR2 is similar in structure and function to TfR1, both receptors have the ability to bind apoTf. TfR2 is shown to have a lower binding affinity than TfR1, based on the increased level of mutations in disorders, such as hereditary haemochromatosis.20 This, therefore, contributes to severe iron overload. As TfR2 has a restricted tissue location, mortality rates for TfR2-induced iron overload are significantly seen to be lower.20
Additionally, research by Kawabata et al.,21 showed erythroid cells had a lower expression of TfR2 and a lower mRNA expression. Thus, suggesting TfR2 may have a pivotal role in iron sensing.
Regulation of cellular iron: The liver also plays an imperative role in the regulation of cellular iron. Where body levels are depleted, iron from the liver is absorbed and utilised in order to maintain physiological balance. There are several molecules involved in this process, including iron regulatory proteins (IRPs) and iron regulatory elements (IREs), which are found in untranslated regions of mRNA sequences.22
IRPs are the proteins that bind to iron-responsive elements in order to maintain iron metabolism (Figure 2). IRP1, for example, is an iron-sulphur cluster protein, which functions by binding to mRNA to suppress translation and degradation.23 IRP2, on the other hand, is similar in structure and function but lacks an iron-sulphur cluster. In conditions of iron depletion, IRE and IRP binding stabilises the TfR1 mRNA, which consequently, increases the uptake of serum iron.24 During this process, the translation of ferritin’s mRNA is inhibited and prevents the storage of iron; which is characteristic of iron overload disorders, such as thalassemia.
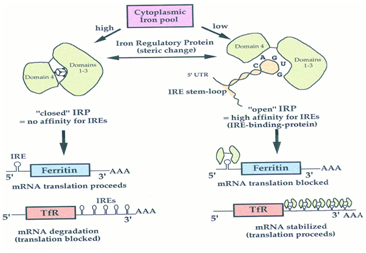
Figure 2 Iron-dependent coordinated control of ferritin and transferrin receptor expression pathway.124
The IRE/IRP biological system also regulates the expression of further IRE-containing mRNAs, which code for proteins involved in iron metabolism. IRP1 and 2 activity is therefore regulated by the post-translational mechanism in response to the levels of iron in the cell.25 Thus, in iron-replete cells, IRP1 accumulates an iron-sulphur cluster, which inhibits IRE binding and triggers the degradation of IRP2. Pantopoulos,25 also suggested that IRP1 and 2 have the ability to respond to iron-independent signals, such as hypoxia or hydrogen peroxide.
Iron storage and recycling: Excess iron taken up by the cell is not always used in the process of metabolism. Hepatocytes found in the liver have the ability to serve as a major iron storage system, through the use of ferritin.26 Ferritin is a ubiquitous intracellular protein that functions to store and release additional iron and as a cytosolic protein found in most tissues, ferritin is, thus, thought to be a prominent biological diagnostic marker for the total volume of stored iron in the body.27 Ferritin’s globular protein complex consists of 24 protein subunits and is the key intracellular iron storage protein in both eukaryotes and prokaryotes. Comprised of a heavy and light chains, the heavy chains function to oxidise ferrous to ferric iron, when bound to ferritin, to keep iron soluble and non-toxic.27
Iron which is stored, however, can be used by those cells which require it for biological processing. This occurs through the help of ferroportin. An increase in exogenous iron levels is also thought to increase intracellular iron levels, which, in turn, increases ferritin expression. Through the co-expression of ferroportin is has been suggested by Nemeth et al.,15 that ferroportin is the likely cause of ferritin degradation and found that mice lacking ferroportin, displayed anaemia and iron overload due to the lowered export of iron from both macrophages and enterocytes.28
Hepcidin
Hepcidin (HAMP): The iron regulatory peptide
Hepcidin: discovery and structure: Despite hepcidin being originally discovered in both human urine and serum, data based on hepcidin expression, regulation and function has been highly established from in vitro studies on mice. Hepcidin (formerly known as LEAP-1) was primarily isolated from plasma ultrafiltrate, but associated with the inflammation of the liver, the anti-microbial peptide was renamed to hepcidin.29 Although mainly synthesised in the liver, traces of hepcidin have also been found in other tissues, such as fat cells.30 The discovery of this peptide, however, led to the finding of hepcidin-induced iron overload disorders.30
The first piece of evidence which linked hepcidin clinically to inflammation came from Hentze et al.,31 where researchers focused on patients with tumours of the liver and microcytic anaemia; both of which did not respond to any form of iron supplementation. They found, in both cases, the tumour tissue to overproduce hepcidin, at a significant quantity. Removal of the tumours, therefore, considerably improved the symptoms of anaemia. From previous research, these discoveries, suggest a highly important role in the regulation of iron by the peptide hepcidin.
Hepcidin, with its gene located on chromosome 19q13.1, is synthesised by hepatocytes as a pre-prohepcidin of 84 amino acids (aa).32 During exportation from the cytoplasm, enzymatic cleavage occurs, producing a 64 aa pro-hepcidin peptide, that is usually found in the endoplasmic reticulum (ER) lumen.33 Through post-translation, the 64 aa peptide is furtherly reduced to produce its mature bioactive 25 aa form.32 Previous analysis by researchers via NMR spectroscopy have found that this cysteine-rich cationic peptide forms a hairpin-shaped molecule with a β-sheet which is bonded by four disulphide bonds.33 Based on this knowledge, hepcidin is thought to structurally resemble other peptides, such as defensins and proteogrin.33
As well as its cysteine-rich form, one of the four disulfide bonds is also thought to be located in the area of the hairpin loop, which suggests a possible domain site, with regards to the activity of the peptide molecule.33 The occurrence of the high cysteine content is similarly believed to be vastly preserved among several established species. Structure and function in vitro and in vivo studies have, thus, shown that its bioactivity is exclusive to the 25 aa peptide. Therefore, suggesting five N-terminal are essential for its mechanistic action.33
This prepropeptide also contains an endoplasmic reticulum targeting signal sequence and a consensus cleavage site for the prohormone convertase.29 In the human body, hepcidin is encoded for by a single gene (HAMP), whose site of expression is primarily in the liver. While close hepcidin homologues have been acknowledged in vertebrates, hepcidin does not appear to be connected to any other formerly known peptide families.34
Function and mechanistic action
Hepcidin is the main regulator of iron metabolism and functions by binding to the iron export protein ferroportin; which occurs on gut enterocytes’ basolateral surface and on the plasma membrane of reticuloendothelial cells (macrophages).35 Primarily, hepcidin breaks down the iron transporter protein, transferrin. By inhibiting ferroportin, this prevents iron from being exported out of the cell and decreases the level of iron being absorbed by surrounding enterocytes in the hepatic portal system and according to Gulec et al.,36 significantly reduces the risk of iron overload within the liver. Inhibition of ferroportin also reduces the release of iron from macrophages. An increased level of hepcidin expression is significantly responsible for a lowered concentration of iron, often characterised by the chronic inflammation of cancer and renal disease.37 As well as its role in iron metabolism, hepcidin has an antimicrobial activity against E. Coli, and other fungal species.37
Mechanistically, hepcidin binds to ferroportin on the cell surface, triggering its internalisation and the subsequent ubiquitination of FPN1. Hence, leads to the degradation of the hepcidin-ferroportin protein complex.38 Previous investigational evidence by Nicholas et al.,39 has confirmed hepcidin’s mechanism of action. It is thought, the disruption of the HAMP gene, contributes to increased expression of ferroportin on the cell surface membrane and hence, it’s characterised symptom of excess iron build-up.
According to Fernandes et al.,40 the binding of both hepcidin and ferroportin is thought involve a disulphide exchange between the disulphide bonds of hepcidin and the exofacial ferroportin thiol residue Cys 326. Patients with C326S mutations were seen to develop early-onset iron overload and the mutant ferroportin was found to have lost its capability in binding to hepcidin. After ferroportin internalisation, the protein complex is degraded and cellular iron export stops.
Additionally, mutations of the HAMP gene, which encodes for hepcidin, is also thought to result in several anaemia, blood-based disorders, such as juvenile hemochromatosis and thalassemia, which in turn, can lead to progression of inflammation and iron-related cancer, such as hepatoma.38
Several animal and human studies have also revealed the primary role that hepcidin plays in iron metabolism. Viatte et al.41 discovered, mice with knockouts of hepcidin were found to produce a model of severe multi-organ iron overload in hereditary hemochromatosis. Likewise, with Nicholas et al.,39 who found transgenic mice that overexpressed hepcidin, died at birth due to iron deficiency anaemia and despite that the regulation of hepcidin is better understood in humans, it is now believed several forms of hereditary hemochromatosis are a result from its deficiency. This is based on the notion that either HAMP mutations or mutations of genes that are speculated to regulate the expression of hepcidin in HepG2 cell lines.42
In summation, hepcidin regulates iron metabolism by restricting the level of iron absorption and release. Thus, reduces the number of iron stores and decreases the availability of iron for surrounding cells and biological processes, such as erythropoiesis and cell cycle phase transitioning.41
Regulation of Hepcidin expression
Hepcidin synthesis is regulated by a vast range of factors, including iron concentration, inflammation, and erythropoiesis. One key process, in particular, related the regulation of hepcidin, occurs through the bone morphogenic protein (BMP) pathway.43 The BMPs are a group of growth factors more commonly known as cytokines and metabologens. These proteins interact with specific receptors on the cell surface and are significantly involved in the signalling of inflammation and neural cell development.44 Several BMPs, including BMP6, have been established to show an effect on hepcidin production, in vivo43 and its knockout, for example, in mouse models is found to result in severe iron overload.45
With regards to hepcidin-induced cancer, the misregulation or absence of the BMP signalling system, for example, is an imperative factor in the development of colon cancer. Over-activation, however, results in the development of adenocarcinoma in the gastrointestinal tract.
As well as its regulation via the BMP pathway, hepcidin synthesis is also regulated in response to the inflammation that occurs in the presence of pro-inflammatory cytokines. One in particular, interleukin 6 (IL-6).46 Increased levels of IL-6, are often the result of inflammatory conditions, such as obesity or inflammatory bowel disease and are thought to stimulate hepcidin transcription through STAT3-dependent mechanisms.15
Links between iron concentration and hepcidin levels have been studied closely over the 10years.47 Erythropoiesis, for example, is a suppressor of hepcidin expression.48 As found in both human and animal studies, serum hepcidin and mRNA levels were significantly reduced, after the administration of erythropoietin.49 Supporting evidence from Tanno et al.50 indicated that two proteins – growth differentiation factor 15 (GDF15) and erythrokine - produced by erythroid precursors, were predominantly involved in hepcidin regulation. GDF15 for instance, has been proven to subdue hepcidin expression, as well as show elevation in patients with thalassemia. Erythrokine, however, is thought to supress hepcidin mRNA in vitro.50
Similarly, the expression of ferroportin can also be regulated independtly from hepcidin, primarily based on cellular iron concentration. As stated by Ganz,51 the concentration of cellular iron is thought to have an effect on both transcriptional and translation aspects of cytoplasmic iron regulatory proteins.
Disorders affecting Hepcidin regulation
Thalassemia: Thalassemia is an inherited autosomal recessive blood disorder which is highly characterised by the abnormal development of haemoglobin (Hb), the protein found in red blood cells that convey oxygen.52 This abnormal development, therefore, leads to the destruction of the red blood cells (RBC) and, in turn, contributes to the prevalence of anaemia.53 As well as its commonly known role in anaemia and inflammation, thalassemia is also thought to be a key contributor to the negative regulation of hepcidin; based on the notion that the disorder directly affects the production of both the alpha (α) and beta (β) chains,54 due to mutations on chromosome 11.55 Hence, thalassemia can be separated into α-thalassemia and β-thalassemia. In addition to this, complex thalassemias as a result from the defective production of all four globin chains (db-, gdb-, and 1gdb- thalassemia), have also been recognised, and clinical research has now suggested that this disorder is similar to other haematology-based diseases, such as haemolytic anaemia and sickle cell disease.55 These clinical variations of thalassemia are currently new potential targets for the prevention of β-thalassemia major and intermedia, which is often the result of homozygosity for both alleles.55
With regards to Hepcidin (HAMP) and thalassemia, hepcidin is thought to negatively regulate iron absorption, degrading the iron exporter ferroportin at the level of enterocytes and macrophages.56 Mutations in the HAMP and FPN1 genes encoding hepcidin and ferroportin are often a result of genetic disorders, such as α or β-thalassemia39 and iron overload is often seen to be the predominant symptom from the decrease in hepcidin synthesis.57 The accumulation of plasma iron decreases a hepatocytes ability to sequester iron and increases the denaturation of ferritin subunits; hence, leads to the ionic release of iron into the blood plasma.58
In haematological blood disorders, such as thalassemia, under production of β-protein chains reduces the expression of hepcidin;59 which in turn, is needed to internalise and degrade ferroportin.60 As a result, reactive oxygen species (ROS) are often produced as a consequence of this oxidative stress,58 where OH radicals are responsible for this DNA and cellular damage. Consequently, mutagenic products are produced, affecting purine and pyrimidine rings and its deoxyribose oxidation products.59 An increase in ROS, therefore, increases DNA mutation levels, gene expression and cell proliferation; while sometimes inhibiting apoptosis.3 Direct oxidative damage to DNA is thus, a common explanation for ROS-induced cancer and immune inflammation.59
Hereditary haemochromatosis: Haemochromatosis type 1 (also referred to as HFE hereditary haemochromatosis or HFE-related hereditary haemochromatosis) is an autosomal recessive hereditary disease characterised by the increased absorption of iron by the gastrointestinal mucosa.61 Attributes of the disorder often show mutations of the HAMP, HFE, transferrin receptor 2 and hemojuvelin genes.42
Distinctive of hereditary haemochromatosis, excess iron is stored in the tissues and organs of the body, including the liver, pancreas, skin, heart and joints.61 As the body has the inability to increase the excretion of iron, iron overload occurs See Figure 3 and causes damage to surrounding tissues and organs.62 It is, for this reason, hereditary hemochromatosis may also be called iron overload disorder.
Mutations of the HAMP and FPN1 genes cause iron overload diseases with autosomal dominant transmission.63 Mutations of these genes, in particular, causes approximately two distinct phenotype abnormalities, which highly depend on the functional state of the protein. One form of the mutation, primarily involves the residues found on the putative cytoplasmic in transmembrane segments (For example, V162del, D157G, G80S and G490D).64 This in turn, leads to the loss in iron exportation and absorption, causing an increased level of iron in macrophages and an elevated serum ferritin.65
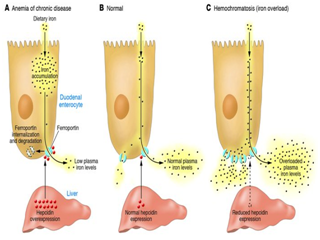
Figure 3 Hepcidin expression.125
A second related phenotype, characteristic of hereditary haemochromatosis, is linked to gain-of-function mutations (C326S/Y for example).65 Evidence from Fernandes et al.40 has specified that this form of mutation is related to ferroportin’s resistance against hepcidin’s mechanism of action. N144D/T and Y64N mutations, in particular, prevent ferroportin’s internalisation, while C326S mutations entirely prevent the binding of hepcidin to ferroportin. It is now thought that C326S mutations lead to the most severe form of iron overload in hereditary haemochromatosis.66
Hepcidin regulation in cancer
Factors up-regulating the expression of Hepcidin in cancer
Hepcidin, inflammation and chronic anaemia: Anaemia is a vastly common haematological irregularity found in cancer patients. Occurring in chronic disease or inflammation, it is believed to be a result of the increased expression of hepcidin, through cytokine signalling (IL-1 or 6 for example).67 Thus, leads to the anaemia associated with iron-induced cancers, such as hepatocellular carcinoma see Figure 4.67
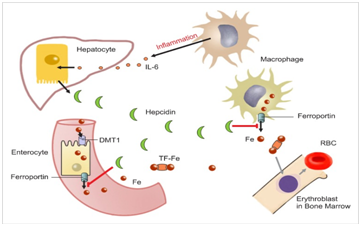
Figure 4 Role of hepcidin in the pathophysiology and diagnosis of anemia.110
From this, it is also thought that infection and inflammation are key regulators of the increased level of hepcidin production. Patients with myeloma, for example, often exhibit considerably raised levels of hepcidin.68 In addition to this, macrophages are also up-regulated in the presence of inflammation. Which, in turn, triggers for the release of the cell signalling proteins, known as cytokines. IL-6 particularly, is known as a primary inducer of hepcidin expression and is thought to inhibit macrophage iron release and absorption in inflammatory states. This is based on the perception that hepcidin production is no longer regulated by iron concentration, but instead, by the stimulation of IL-6.68
Evidence from Peyssonnaux et al.69 demonstrated that during inflammatory states, serum iron up-regulated the expression of hepcidin synthesis in participants and was coined as an induction signal for its production. This was also found to affect the serum transferrin saturation. As well as this, they found, hepcidin could also be triggered for production by myeloid cells, due to the activation of TRL4, which is a receptor found on macrophage membranes.
JAK-STAT pathway: Hepcidin is also highly influenced by the interactions of the JAK-STAT pathway. This is one of the primary signalling pathway involved in the progression of cancer and is seen to involve a receptor-associated Janus kinase (JAK) and a signal transducer and activator of transcription (STAT).70 The pathway, itself, is vital for the regulation of both hepcidin and ferroportin and coincidently, their dysregulation; which is thought to play a role in the anaemia and iron overload characteristic of several cancer types, like myeloma or hepatocellular carcinoma.71
Upon the binding of a cytokine (IL-1 or 6, for example) to its cell-surface receptor, dimerisation and activation of a JAK tyrosine kinase can occur.71 The tyrosine residues on the receptor can then be phosphorylated by the presence of these activated JAK molecules. As well as JAK phosphorylation, the signal transducer STAT molecules are also phosphorylated, leading to the dimerisation and the activation of gene transcription.72 The mechanistic action of this pathway, however, is highly important in the diagnosis of several epithelial tissue and haematological cancers.
Away from the basic mechanistic action of the JAK-STAT pathway, ferroportin is known as the primary component of iron transport and metabolism. As previously established, hepcidin regulates the activity of ferroportin by triggering its internalisation and degradation. A study by Ross et al.73 has reported this concept is highly dependent on JAK2 activation, the phosphorylation of ferroportin’s tyrosine residues 302 and 303 and STAT3’s phosphorylation, which is vital for the process of transcription. Despite, previous knowledge, suggesting for both hepcidin and ferroportin up-regulation, Ross et al.73 have also suggested that there is not definitive link between ferroportin expression and the JAK-STAT pathway. Thus, meaning a clear potential therapeutic role in hepcidin up-regulation.
Bryan et al.74 however, has disregarded this theory, suggesting in hepatocellular carcinoma, a secondary molecule must be present in order to trigger the expression of hepcidin. One example, in particular, is thought to occur through the activation of STAT3 by the inflammatory cytokine IL-6. Throughout their study, Bryan et al.74 found that the binding of IL-6 to its receptor, lead to the activation of JAK2, which in turn, phosphorylates STAT3. STAT3 was then translocated to the nucleus and bound to its binding motif at -64/-72 within the promoter region of hepcidin. Thus, leading to the induction of hepcidin’s transcription and production Bryan et al.74
De Domenico et al.75 however, also reported that JAK2 activation and ferroportin phosphorylation are vital for hepcidin-mediated FPN1 internalisation. They carried out a study which focused on the expression of mRNA in FPN-expressing macrophages exposed to different concentrations hepcidin. The investigation showed that gene expression was a result of hepcidin and not the downstream activation of relevant genes. As well as this, De Domenico et al.75 also suggested as role for hepcidin as a transcription mediator for several other genes. They confirmed that increased mRNA expression was due to the binding of hepcidin and ferroportin and that STAT3 was the necessary promoter for this mechanistic action. With regards to cancer, they suggest novel therapeutic roles of small interfering RNA (siRNA) that significantly increased FPN1 silencing. By silencing FNP1, the exportation of excess iron is considerably reduced, resulting in the decreased formation of NTBI’s. There was evidence to support this based on the down-regulation of the androgen-induced 1 and prostate transmembrane proteins.
Continuing from De Domenico et al.,75 they also discovered that hepcidin is involved in the FPN/JAK2/STAT3-dependent anti-inflammatory negative feedback loop. FPN-expressing macrophages showed a down-regulation of IL-6 and tumour necrosis factor α (TNF-α), when exposed to the siRNAs. This lead to a suppression of cytokine signalling 3 (SOCS3), a negative regulator of the JAK-STAT pathway. Given this potential role of JAK2 and its inhibitors, the therapeutic stabilisation of ferroportin through the inhibition of JAK2 is now a possibility for ferroportin down-regulation mechanisms, despite the lack of evidence for its role in the prevention of ferroportin internalisation.
On the other hand, Sharma et al.76 has concurrently omitted this and has stated that TNF-α is also known to promote ferritin synthesis. Thus, increasing the body’s total iron store. Additionally, this has been linked to the STAT3 and SMAD4 pathways, whereupon signalling, hepcidin-mediated inflammation occurs. This type of inflammation has now been connected to myeloma and adipose tissue neoplasm.75
In cancer patients, who showed increased levels of hepcidin, also displayed increased levels of IL-6. Over activation of the JAK-STAT pathway is thought to be a contributing factor to hepatocellular carcinoma, by preventing the occurrence of cell death – apoptosis – and cell cycle checkpoint regulation77 Similarly, interferon-γ, an activator of the JAK-STAT pathway, provides a link between the immune systems response to iron. Cancer patients, who showed increased levels of hepcidin, also showed highly up-regulated levels of lipocalin 2. Connected to a deficiency in the HFE gene, hepcidin expression was signalled for production, as well as upstream stimulatory factors, USF1 and USF2.78 The overexpression of these stimulatory factors, have the ability to prevent c-Myc-dependent cellular proliferation, through inhibition and this anti-proliferative role suggests a high implication for hepcidin in carcinogenesis.
MAPK pathway: Connected to the JAK-STAT pathway, is the mitogen-activated protein kinase (MAPK) pathway. These MAPK pathways consist of three kinase molecules, which are activated upon phosphorylation by mitogen-activated protein kinase kinase (MAPKK). Once activated by its phosphorylation from a MAPKKK protein,79 hepcidin is also believed to promote osteogenic differentiation through The MAPK pathway in mesenchymal stem cells and that over-activation is thought to lead to osteosarcoma.80
With limited research available on the current topic, Lu et al.,80 investigated the effects of hepcidin on the osteogenic differentiation of mesenchymal stem cells (MSCs). They hypothesised that the MAPK/p38 signalling pathway may play a significant role in the process of osteogenic differentiation. By adding 10μM of SB203580 - an inhibitor of the MAPK/P38 pathway - they found that hepcidin considerably up-regulated the level of P38 and, therefore, may potentially have a key role as a sensor for oxidative stress in the process of carcinogenesis. Based on this, they reported that a deficiency of P38 lead to increased proliferation of tumour cells and decreased apoptosis. Backing up Lu et al.,80 Kim et al.,81 has also suggested that, cells treated with the SB203580 inhibitor, showed significantly decreased levels of osteoblast differentiation and inhibited hepcidin production. The data from both studies, thus, demonstrates that the promotion of hepcidin-induced osteogenic differentiation may primarily be mediated by the MAPK/P38 pathway and have an imperative role in the progression of cancer.
IL-6 and HIF-1 toxicity: IL-6 is a pro- and anti-inflammatory cytokine, encoded for by the IL6 gene, which functions to promote survival and apoptotic signals, along with cell proliferation.82 Secreted by both T cells (Th cells) and macrophages, an immune response be stimulated via the ability to function with protein kinase cascades.70 In addition to signalling, IL-6 also acts upon lymphocytes, which includes B cells, to encourage auto-antibody production and naïve T helper cells, to promote Th17 cell differentiation.83 The IL-6 receptor is also highly important, because of its heterodimeric complex (involving two Ig-like containing proteins – gp80 and gp130).84 Due to the increasing variety in the amount of inducers for IL-6 secretion, such as IL-1 β or tumour necrosis factor- α (TNF-α), many cell types have the ability to respond to IL-6 signalling. Which, in turn, gives a greater understanding into the mechanistic actions of cancer signalling pathways.85
In line with this, immunologists are highly interested in IL-6’s mechanistic role with regards to carcinogenesis. While the presence of IL-6 in tissues is not an irregular occurrence, its increased expression is thought to lead to the chronic inflammation linked with numerous types of cancer.86 In addition, in the presence of oxidative stress, IL-6 also has the capacity to be over-expressed in cancer patients. They are in fact found to have raised levels of IL-6 in their serum, making this an imperative biomarker for chronic inflammation.72
With regards to hepcidin, and as mentioned before (See 2.2), IL-6 activates hepcidin’s expression, primarily through the JAK-STAT pathway. Vaupel et al.87 has hypothesised that the inflammatory cytokine IL-6, is thought to directly mediate hepcidin through the binding of a STAT3 molecule. Characteristic of cancer, mutations alter the normal functional of cell growth and pathway signalling and relating to IL-6, this often leads to the production of tumour hypoxia - where tumour cells are deprived of oxygen. As well as this, they suggest that the under production of hepcidin results in the increased formation of non-transferrin bound iron molecules (See 3.2.1), which, conversely, increases the toxicity and in some cases produces reactive oxygen species (ROS) - a consequence of this oxidative stress.58 As a result of this DNA oxidation it could be thought that mutagenic products may directly affect the purine and pyrimidine rings, as well as deoxyribose oxidation products.59 An increase in ROS, may, therefore, raise DNA mutation levels, gene expression, cell proliferation and in some cases, inhibit apoptosis.88 Direct oxidative damage to DNA is thus, a common justification for ROS-induced cancer and immune inflammation.59
Lesbordes-Brion et al.89 has simultaneously proposed that oxidative damage from ROS will only take place at particular concentrations of hepcidin, in both deficient and excessive states. As hepcidin has a subsequential role in iron homeostasis, both situations are shown to prompt cell proliferation and reduce the transitional movement through the cell cycle.
Linking back to toxicity, from the under-production of hepcidin, histotoxic hypoxia, or hypoxia, may occur when the oxygen supply is greatly reduced due to the increased presence of a cytotoxic metal.90 Conventionally, in hypoxia, there is a greater production of the hypoxia-inducible factor (HIF-1), which contains the HIF-1α and HIF-1β subunits – These are the fundamental regulatory transcription factors necessary for cellular changes.91 According to Dang et al.91 humans have presented the up-regulation of IL-6 genes and hence, an increased hepcidin expression, due to the induction of HIF-1. When linked to metabolism, HIF-1 is, therefore, thought to have a protective role, affecting glycolytic genes to deal with the changes in iron concentration and oxygen availability.
Contrary to this, Liu et al.92 has alternatively stated that hypoxia may be a key player in the suppression of hepcidin. Thus, could possibly increase cellular iron uptake / absorption and its release from the iron store known as ferritin.
Down-regulation of hepcidin production in cancer
Despite finding that hepcidin is up-regulated in patients with cancer; it is thought that there are inhibitory mechanisms, which may negatively regulate the expression level of hepcidin instead; some of which include: erythropoietin in HepG2 cell lines and via the bone morphogenetic protein (BMP) pathway.
Non transferrin bound iron: Iron primarily circulates in the plasma and is bound to transferrin, a transporter protein, which is vital for iron-dependent processes, such as iron metabolism or erythropoietin.93 In hereditary haemochromatosis for example, iron overload often results in the increased level of circulating iron which does not have the ability to bind to transferrin or ferritin, due to transferrin’s binding capacity.94 NTBI’s, however, are taken up by the liver more readily when compared to transferrin bound iron.95 Based on this notion, Breuer et al.96 has suggested that the uptake of NTBI’s by hepatocytes is vital in preventing the formation of free radicals, which in turn, causes damage to surrounding cell types. Despite this, the threshold at which a hepatocyte can take up this excess iron, is relatively low and increased iron accumulation may become toxic. Thus, leads to the development of liver related disorders, such as hepatocarcinoma – Similarly backed up by Brissot et al.97 This condition, in turn, is, therefore, a characteristic of both beta-thalassemia and hereditary hemochromatosis.97
It is thought T lymphocytes are highly exposed to excess circulating iron, based on its pivotal role as a cellular component of peripheral blood. Acting as a physiological barrier against iron-mediated toxicity, evidence from Sousa et al.98 suggests a deficiency in CD8+ T cells is linked to the increased uptake of NTBI. As well as this, there is a supposed negative correlation between total iron stores of the body and circulating CD8+ lymphocytes, in HFE-hemochromatosis patients.99 Thus, supporting a potential role in the protection against excess iron build-up.98
Despite this ROS producing role, Anderson et al.,9 has suggested a mechanistic overview which states at high levels of transferrin saturation, hepcidin regulation is reduced. They found in rats, with an acute-phase response to Freund complete adjuvant (FCA), an increased hepcidin expression trigger the decreased in intestinal iron transporter expression. As transferrin saturation increased, hepcidin expression decreased, observing that changes in hepcidin expression may be a potential new biomarker for iron overload-induced liver cancer diagnosis.
However, under severe conditions of iron overload, the regulation of hepcidin expression appears to be a more complex process. Weinstein et al.100 for example, treated mice with iron-dextran, phenylhydrazine (PHZ), an inducer of acute haemolysis, which inhibits hepcidin expression. The same was done for hypotransferrinemic mice. This model of iron overload, presenting increased levels of non-transferrin bound iron, displayed lower hepcidin levels, as well as a significant increase in hepatocyte iron loading. Both haemolysis and hypotransferrinemia, present with high levels of NTBI and thus, can lead to the formation of HAMP mutations and ROS production.
It could be concluded, that the occurrence of NTBI’s is therefore, highly dependent on up- or down-regulation of hepcidin. Occurring primarily in cancer patients with symptomatic iron overload or iron deficiency, hepcidin regulation, is therefore, believed to be mainly influenced by inflammation, the JAK-STAT pathway, cytokines and the BMP.98 Therapeutically, this has also provided a potential new alternative to cancer therapies, such chemotherapy, targeting signalling pathways, involved in the differentiation of cancer cells.
Erythropoietin and HAMP suppression in HepG2 cell lines: Erythropoietin (EPO), a glycoprotein hormone, is thought to have a direct effect on the transcription of hepcidin. Previous studies, looking at the role of hepatocytes and HepG2 cells have found that EPO, is a common regulator of HAMP expression via the EPO receptor (EPOR).101 Other studies however, such as Pak et al.102 and Lakhal et al.103 have alternatively proposed that the use of EPO in cancer patients, is seen to supress HAMP within the liver, resulting in cancer-related iron overload. Both studies found that growth differentiation factor 15 (GDF15), was secreted at high levels from matured erythroblasts and, in turn, suppressed the transcription on HAMP in both hepatocytes and liver hepatoma cells.
Linking back to thalassemia, Tanno et al.50 proposed that serum GDF15 levels in cancer patients suffering from the disease. It is thought, that GDF15 suppresses hepcidin during erythropoiesis and causes this to fall into an ineffective state. Only in cancer patients, is this evidence, relevant, primarily due to the notion that in other anaemic situations, high levels of EPO and GD515 were less apparent. Thus, this mechanism, has provided the possibility that GDF15 may be a marker of ineffective or apoptotic erythropoiesis104 in cancer patients.
A study by Leyland-Jones,105 similarly, investigated the effects of EPO-based treatments in anaemic breast cancer patients and their survival rates. A higher mortality rate was observed in patients being treated with Eprex, an EPO, than compared to those treated without. This has led to the concern that a raised level of EPO may, in fact, have adverse side effects on the survival of patients with cancer. A related follow-up study Henke et al.,106 on head and neck cancer patients with anaemia, found that while EPO did have a positive effect on the treatment of anaemia, it failed to prevent the progression of cancer, as found with Pinto et al.101
Bone morphogenetic protein pathway: As mentioned previously (2.2), hepcidin regulation is mediated via the bone morphogenetic protein (BMP) pathway, in response to the plasma transferrin levels.74 Bryan et al.74 has proposed, that BMPs – primarily BMP6 – is the sole mediator for hemojuvelin’s (HJV) response to cellular iron concentration. Going against, what was found in the results, they suggest cancer patients actually have lowered levels of hepcidin, based on the subsequent interactions of SMAD4 and phosphorylated SMAD7. It is thought this interaction is a negative regulator for hepcidin expression, as it binds directly to the promoter region to repress its transcription. As well as SMAD7 interactions, they found an increased level of the transmembrane protease serine 6 (TMPRSS6) in hepatocellular carcinoma, which inhibits the BMP pathway to prevent a signalling cascade. Overall, disregarding the increased hepcidin levels, they propose SMAD7 interactions and inhibition of the BMP pathway may have a therapeutic role in the prevention of iron deficiency in cancer patients.74
Therapeutic and clinical applications of Hepcidin
Overview
Having established the primary role of hepcidin in iron homeostasis and cancer progression, treatment for iron overload and deficiency could be considered to be harmful, based on the notion, that it has the ability to trigger neoplastic transformation and contribute to the activation of cancer-related T-cells.107
Despite the lack of therapeutic purposes of hepcidin in human models, on-going studies conducted on mice, have discovered potential therapies which could be used to prevent of improve the accumulation of iron in cancer patients. One main focus in particular, is the use of hepcidin agonists and antagonists.108
Other potential drugs, have been found to target the regulation of hepcidin expression, including dorsomorphin and soluble HJV, which act as BMP signal antagonists.108 As well as these antagonists, the use of cytokines, such as IL-22 have been suggested to have a suppressive effect on hepcidin production and improve the incidence of anaemia in iron-induced cancer cases.109 Hydroxyproline inhibitors may also be highly effective in the inhibition of hepcidin, triggered by erythropoiesis and by hypoxia-inducible factors.110
IL-22 as a hepcidin suppressor
Interleukin-22 (IL-22), similar to IL-6, is a cytokine encoded for by the IL22 gene,111 which produces pro-inflammatory responses in response to activated T cells.112 Evidence from Lim et al.113 has shown that the dysregulation of IL-22 is most commonly found in patients with hepatocellular carcinoma.
Because IL-22 is restricted to tissue epithelial cells, rather than immune cells, it is thought to be an active target for cancer therapy and symptomatic iron overload disorder.114
Kryczek et al.115 detected that in patients showing increased levels of hepcidin expression, also displayed a higher level of IL-22. Which is thought to be predominantly produced by CD4+ T cells. In a mouse model, looking at expression levels in a HepG2 cell line, both IL-22 and hepcidin required the chemokine receptor CCR6 and its ligand CCL20, to promote the activation of STAT3.
Smith et al.116 also backed this up, to suggest that IL-22 influences hepcidin production upon the activation of JAK2 and STAT3. Mouse who were injected with IgG1 Fc attached to the N-terminus of IL-22- an IL-22R agonist – were seen to have an increased expression of hepcidin and a subsequent decrease in the level of circulating serum iron. Based on this notion, IL-22 may be a new therapeutic target for cancer prevention, using an Ab-mediated blockade of JAK-STAT signalling. This Ab-blockade, while useful in cancer inhibition, also partially blocks hepcidin expression, thus, playing a role in the treatment of cancer-induced anaemia.116 As well as its role in anaemia and iron overload disorder, IL-22 is thought to be protective against liver necrosis in mouse models.116
Hepcidin agonists
Minihepcidins: Minihepcidins are peptide-based agonists, which are designed based on the region of hepcidin that interacts with ferroportin.117 In order to confirm the clinical uses of minihepcidins, Ramon et al.,118 tested PR65 – a type of minihepcidin - in a group of knockout mice, who had severe hemochromatosis. During the preventive testing, PR65 was seen to fully inhibit iron loading in the liver. At high doses of PR65, anaemia was also significantly reduced. In treatment mode however, knockout mice with pre-existing iron overload were exposed to PR65 for a period of two weeks. Ramon et al.118 found a partial relocation of excess iron to spleen and concluded that minihepcidins may be useful in the prevention of iron overload often found in HFE and thalassemic patients.
Targeting TMPRSS6: Nai et al.119 on the other hand, has suggested that targeting TMPRSS6 may be a more efficient way of treating hepcidin-induced anaemia in cancer patients and iron overload. Nai et al.119 demonstrated the inactivation of TMPRSS6 increased hepcidin levels, decreased iron overload states and increased the efficiency of EPO. From this, it was suggested that TMPRSS6 is a negative regulator for hepcidin expression in both mouse and human models.
Schmidt et al.120 similarly, used siRNAs against TMPRSS6. Double-stranded siRNA nucleic acids are designed to prevent the expression of target genes through the RNA interference pathway. The study, aimed to evaluate the effect of TMPRSS6 knockdown in hepatoma mice models. After six weeks of treatment, they found a significant reduction in the concentration of liver serum iron and an increased level in the spleen. As well as this, treatment improved the anaemia found in cancer-induced hepcidin overexpression. Schmidt et al.120 consequently concluded, that targeting TMPRSS6 may be a favourable approach to treating iron overload and iron deficiency anaemia.
Additionally, research related to TMPRSS6, has led to potential new specified therapeutic treatments, targeting certain signalling pathways see Figure 5. Despite the lack of strong evidence, the use of agonists and antagonists to regulate hepcidin expression, may provide a clear path for future research in this area.63
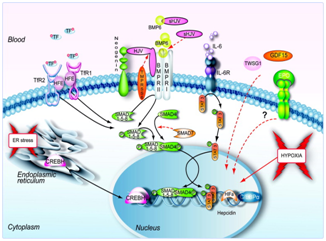
Figure 5 Signals and pathways controlling hepcidin expression in the liver.63
Hepcidin antagonists
Spiegelmers: The hepcidin antagonists, spiegelmers, are RNA-like oligonucleotides with L-stereochemistry, which provides resistance to nucleases. Similarly known as an anti-hepcidin, used in the treatment of hypoxia and the anaemia of cancer, a study by Schwoebel et al.,121 looking at the effects of the spiegelmer NOX-H94 on inflammation-induced anaemia in cynomolgus monkeys, has provided a clinical application for the inhibition of hepcidin. Cynomolgus monkeys, injected with IL-6 for a week, were found to display high levels of anaemia. As part of treatment, using NOX-H94, cynomolgus monkeys showed a reduced level of anaemia in both phase I and II of the trials. Despite this, the main task for the efficacy of anti-hepcidin may be based on the high rate of hepcidin production. Xiao et al.122 estimated that hepcidin production in humans is likely to exceed 12 mg per day. Based on this, it could be suggested that inhibitors of hepcidin, may lessen the normal clearance process of hepcidin and thus, cause further hepcidin accumulation. With little evidence to support Schwoebel et al.,121 further research would need to be addressed and produce agents with a potential shorter half-life. As well as this, for use of antibodies, the must have the ability to be recycled after playing a role in hepcidin’s degradation. Furthermore, partially blocking hepcidin activity may increase the level of iron delivery to the bone marrow, where it will potentially improve erythropoiesis. Again, further clinical trials are needed for this intended use of therapeutic treatment.123–125
Despite iron’s pivotal role in cellular iron metabolism, over or under expression of hepcidin, the primary iron regulatory molecule, is seen to lead to cases of ROS-induced cancers, such as hepatoma. With ongoing research, looking at the regulation of hepcidin expression in primary liver cell lines, such as HepG2, therapeutic treatments have been focused on targeting the signalling pathways which play a role, in not just hepcidin’s production, but as well as ferroportin’s. Studies show that in both under or over expression, hepcidin has an effect on the progression of carcinogenesis. In cases of under expression, increased levels of serum iron, trigger the production of ROS, which is simultaneously thought to play a major role in cellular DNA damage, as well as in conditions, such as hypoxia. Over expression, however, increases the level of cellular iron build-up, due to the over internalisation of ferroportin. As hepcidin has a subsequential role in iron homeostasis, both situations are shown to prompt cell proliferation and reduce the normal biological functioning of certain signalling pathways.
Additionally, with functional roles in the signalling pathways, such as the JAK-STAT pathway and the BMP pathway, targets for hepcidin regulation have been established over the last 20 years, suggesting a further significant role in cancer. Regulation by EPO, agonists and antagonists, such as minihepcidins and spiegelmers, has also shown a suggested therapeutic use, which targets hepcidin regulation in both anaemic and iron-overload cancer patients. Regardless of thorough research, there is still little evidence to provide a definite use on clinical terms. Which proposes, further clinical trials are needed for the intended use of therapeutic treatments against hepcidin in cancer.
None.
The authors declare there is no conflict of interests.
None.

©2015 White. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.