Journal of
eISSN: 2373-633X


Research Article Volume 14 Issue 1
1Senior Researcher of Nacional Cancer Institute (INCA), Brazil
2Resident of the 3rd year of Radiology at INCA, Brazil
3Physician at the National Cancer Institute and Coordinator of Radiology in Clinical Research, Radiologist at Nacional Cancer Institute (INCA), Brazil
4Master in Labour Economics, UFPB, Statistician of the Complexo Hospitalar Mangabeira, Brazil
5INCA Anesthesiologist, Responsible for the CET-SBA of the National Cancer Institute, Brazil
6INCA Anesthesiologist, Co-Responsible for the CET-SBA of the National Cancer Institute, Brazil
Correspondence: Dr. Luiz Eduardo Imbelloni, Senior Researcher of Nacional Cancer Institute (INCA), Av. Epitácio Pessoa, 2356/203 – Lagoa, 22411-072- Rio de Janeiro, RJ – Brazil, Tel + 55 11 99429-3637
Received: February 04, 2023 | Published: February 15, 2023
Citation: Imbelloni LE, Cardoso BB, Torres CC, et al. The anatomy of the thoracic spinal canal investigated with magnetic resonance imaging in children aged 0 to 13 years old. J Cancer Prev Curr Res. 2023;14(1):15-22. DOI: 10.15406/jcpcr.2023.14.00512
Background: Anesthesiologists frequently give thoracic epidural blocks for a variety of surgeries. Magnetic Resonance Imaging (MRI) has provided studies of the nerves (cauda equina) inside the dural sac. But only a few correlate the spinal cord with the dura mater in the thoracic region. The aim of this study was to evaluate four measurements between the skin and the spinal cord in three thoracic segments.
Method: Retrospective study with 105 children aged 0 to 13 years in the supine position underwent MRI. Medial sagittal slices of the 2nd, 5th, and 10th thoracic segments were measured for the relative distances through two devices from the Radiology Service. The following parameters were evaluated: spinal dura mater and the spinal cord distance; skin to spinal cord distance; needle entry angle between the skin and intervertebral space; and distance corrected by an angle between spinal dura mater and the spinal cord. For statistical analysis were used the following tests Chi-Square, Wilcoxon, Friedman, ANOVA, and Krukal-Wallis, and the significance level was set at 0.05.
Results: The posterior dural-spinal cord distance is significantly greater in the midthoracic region (5th thoracic=5.61±1.61 mm) than at upper (2nd thoracic=4.52±1.25 mm) and lower thoracic levels (10th thoracic=4.68±1.38 mm) (p<0.015). There were no differences between interspaces T2 and T10. There was no correlation between the age and the measured distance between the dura mater and the spinal cord. The entry angle of the needle at T2 has observed a value of 27.16±5.12 degrees, at T5 was 38.68±6.10 degrees and at T10 was 39.08±5.23 degrees. These angles reflected an increase in the distance between the SDM-SC in the three evaluated segments (T2=34%, T5=45%, T10=40%), reflecting in greater protection of the spinal cord.
Conclusions: This study demonstrated there is a greater depth of the posterior subarachnoid space at T2, T5, and T10 levels. The greater distance was found at T5. The correction of the entry angle of the needle increased the distance in the three interspaces. This distance between the dura mater and the spinal cord protects the spinal from damage in case of an accidental dural puncture during thoracic epidural attempts. It certainly will permit a spinal needle to provide segmental thoracic spinal anesthesia without damage.
Keywords: anatomy, intrathecal, MRI,neuraxial, spinal cord, thoracic spinal cord, pediatric, children
SDM–SC, spinal dura mater and the spinal cord distance; Skin–SC, skin to spinal cord distance; Angle–Degree, needle entry angle between the skin and intervertebral space; DcAn–SDM–SC, distance corrected by angle spinal dura mater and the spinal cord; MRI, magnetic resonance imaging
Question: A retrospectively investigated the distance from the dura mater to the spinal cord (SDM-SC), the distance from the skin to the spinal cord (Skin-SC), the angle of entry of the needle between the skin and the intervertebral space (Angle-Degree), and angle-corrected distance of the spinal dura mater and spinal cord (DcAn-SDM-SC) in the three thoracic segments (T2, T5, T10), analyzing the MRI of children aged 0 to 13 years, without spinal or spinal cord disease, were studied to explain the lack of injury during drilling of the thoracic epidural and possibility of performing thoracic spinal anesthesia in children.
Findings: The mean dura mater to spinal cord distance was 4.52 mm at T2, 5.61 mm at T5, and 4.68 mm at T10, and the mean distance corrected by angle spinal dura mater and the spinal cord was 6.07 mm at T2, 8.18 mm at T5, and 6.57 mm at T10.
Meaning: The correction for the angle of entry of the needle into the intervertebral space significantly increased the distance between the dura mater and spinal cord, and it will certainly allow a spinal needle to provide segmental thoracic spinal anesthesia or accidental technical epidural puncture without neurological damage.
Leonardo da Vinci's anatomical work lies halfway between scientific and artistic interests. He held more than 20 cadaver dissections in medical schools, describing magnificently the spine.1 For some time there has been the possibility of studying anatomy even in living people, through imaging techniques such as radiography, endoscopy, angiography, computed axial tomography, positron emission tomography, imaging magnetic nuclear resonance, ultrasound, thermography, and among others.2 The anatomical and imaging features differ between the thoracic and lumbar spine.
Each step of the evolution of spinal anesthesia, which allowed us to move from the anatomy of the cadaver to the technology of studies of Magnetic Resonance Imaging (MRI), is explained by apparently random factors that are connected to the development of technology and history. Every step in the evolution of the world is always the result of events and people who came before and never imagined, for better or for worse, the future consequences of these acts.
MRI is the most significant technological advancement in the diagnostic examination of the spine. To better understand and predict the MRI appearance of pathologic processes involving the spine, one should have a basic understanding of the normal MRI anatomy. At around two years old, the spine begins to show its normal curvatures.3 The ossified portion of the vertebral body increases substantially in size and begins to assume its adult appearance, with almost complete ossification of the pedicles and articular processes. The disc space and nucleus pulposus become longer and thinner. Thus, our study will be carried out with normal MRI of children between birth and 13 years of age. By age 10, the curvature of the spine is already similar to that of an adult.3
Recently, a retrospective study was performed with 99 MRIs to determine the rate of incidental findings of the cervical, thoracic and lumbar spine in the pediatric population, concluding that MRI is a useful modality in pediatric patients with scoliosis or pain complaints.4 Another retrospective study of 190 spinal MRIs of patients up to 18 years of age, showed that the frequency of incidental findings on spinal examinations in the pediatric population is lower than in the adult population.5 In another study on children and adolescents, it is believed that knowing the slopes of the sagittal plane of the development of the spine in childhood and adolescence will contribute to the earlier determination of pathologies.6
The anatomy of the thoracic spinal canal in different postures was evaluated in 9 volunteers adults showed that the distances between the the dura mater and spinal cord were wider in the mid-thoracic region and with the subject in the sitting position (head-down).7 In fact, no published article was found on the distances of the spinal cord membranes and the space between the thoracic dura mater and the spinal cord membrane in children. Several publications have already evaluated these measures in adult patients.7-10 These studies allowed the explanation of the lack of spinal cord injury during accidental perforation in epidural anesthesia and the use of thoracic spinal anesthesia.
The anatomical characteristics of the thoracic and lumbar spine are significantly different. In this retrospectively investigated the distance of the dura mater to the spinal cord (SDM-SC), skin-to-spinal cord distance (Skin-SC), needle entry angle between the skin and intervertebral space (Angle-Degree), distance corrected by angle spinal dura mater and the spinal cord (DcAn-SDM-SC) at the three thoracic segments (T2, T5, T10), analyzing the MRI of children aged 0 to 13 years, without spinal or medullar disease, to check if there is a possibility of performing thoracic punctures for spinal anesthesia in children.
A retrospective study approved by the Ethics and Research Committee under number 0867/2009 for the evaluation of the thoracic and lumbar spine by MRI in adults and extended to children between 0 and 13 years old. As this is a retrospective study, the waiver of the Free and Informed Consent Form was requested and approved.
Between April 1, 2019, to June 30, 2022, selected to evaluate the MRI performed at the Cancer Hospital (HC 1) of the National Cancer Institute (INCA), and the MRI was evaluated in the database of the Department of Radiology. All healthy children were examined supine with the MRI 1.5-T superconducting system scanner (Gyroscan Intera, Philips Medical Systems, Best, the Netherlands) or Magneto Symphony 1.5-T Siemens. Each MRI exam complied with the protocol established by the radiology service and evaluated patients with different clinical conditions. The results obtained were stored in a digital imaging system.
The inclusion criteria for this research were child patients of both sexes, aged between 0 and 13 years, who did not present any pathology of the cervical, thoracic and lumbar spine and/or spinal cord. Children's demographic data were recorded as gender and age. The children studied were divided into two age groups: group 1, children aged 0 to 6 years, and group 2, children aged 7 to 13 years.
The images of the thoracic spine were performed using the Spin-Echo sagittal slice. In one of the MRI images of the two groups of patients studied (Figure 1) that covered the entire spine (C1 to L5), the relationships between the spinal cord and the vertebrae in the interspinous spaces of T2, T5, and T10 were evaluated. The measurements were evaluated in the 2nd, 5th, and 10th thoracic vertebral segments. In the three thoracic intervertebral spaces, the distance between the spinal dura mater and the spinal cord (SDM-SC), the distance between skin to spinal cord (Skin-SC), the needle entry angle between skin and intervertebral space (Angle-Degree), and the distance corrected by angle spinal dura mater and the spinal cord (DcAn-SDM-SC) in a horizontal position perpendicular to the spine was measured. Each space was measured three times and the mean value between these measurements was calculated. In all patients, the needle entry angle necessary to reach the subarachnoid space in the interspinous spaces of T2-T3, T5-T6 and T9-T10 was measured, using the protractor tool available in the Carestream PACs image viewer. After obtaining the angles for entry of the spinal needles into the three spaces, the distance between the spinal dura mater and the spinal cord was again calculated, correcting the angle of entry of the needle into the intervertebral space and obtaining its measurement. Each space was measured again three times and the mean value was calculated.
Statistical analysis
To analyze the association between categorical variables, we used the Chi-Square test. To analyze the association of interval variables between independent samples, we used the Wilcoxon test and the Friedman test, which are non-parametric versions of the ANOVA. To analyze the association of interval variables between paired samples, we used the Kruskal-Wallis test. For all tests considered, the significance level is 0.05.
In the period from April 1, 2019, to June 30, 2022, 21,392 MRIs were performed at the hospital. Using patients born between April 1, 2006, and June 30, 2022, we found 3,055 MRIs. Using the following terms as filters: dorsal column, thoracic column, total column, spinal cord compression, and neuroaxis 252 MRI exams were selected for the research. MRI exams that presented the following terms were excluded: intradural, intramedullary, or extramedullary lesions; and/or extradural injuries with spinal cord compression; and/or exams where the skin has been cut in the image; and/or patients over 13 years of age; and/or exams of the same patient, resulting in 105 selected exams (Figure 2).
In the study, period was retrospectively evaluated, with 60 male and 45 female children. Children's demographics are shown in Table 1. Regarding the two age groups studied, the results for age and sex incidence are shown in Table 2.
|
Variable |
Children |
|
Number |
105 |
|
Age (years) |
7.03±3.32 |
|
Weight (kg) |
26.23±14.02 |
|
Height (cm) |
119.77±22.92 |
|
Gender: F/M |
45/60 |
Table 1 Patients’ characteristics regarding age, weight, height, and sex (Mean±SD)
|
Variable |
Group 1 (0-6 years) |
Group 2 (7-13 years) |
p-value |
|
Age (years) |
3.89±1.60 |
9.62±1.74 |
0.0000* |
|
Female (n) |
26 |
23 |
0.57172** |
|
Male (n) |
22 |
34 |
|
Table 2 Patients’ characteristics regarding both groups (Mean±SD)
MRI results of a patient with all measurements evaluated in the three segments and the increase calculated by the entry angle of a needle (Figure 3).
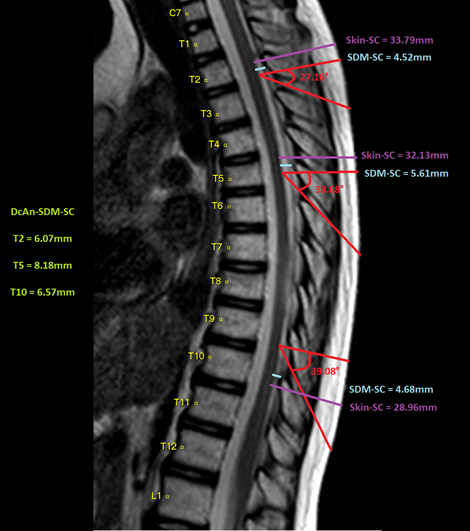
Figure 3 Results of measurements in the three thoracic segments and value corrected by the needle entry angle.
The distance between the spinal dura mater and the spinal cord is significantly great in the middle thoracic region (5th thoracic segment) than at upper (2nd thoracic segment) and lower thoracic level (10th thoracic segment) (Table 3). The Friedman test suggests that there is a significant difference, returning a p-value close to zero. The graph indicates which differences are significant (p-value < 0.05).
Regarding the distance from the skin to spinal cord (Table 3). The Friedman test suggests that there is a significant difference, returning a p-value close to zero. The graph indicates which differences are significant (p-value < 0.05).
Regarding needle entry angle between skin and the three intervertebral spaces. The Friedman test suggests that there is a significant difference, returning a p-value close to zero. The graph below indicates which differences are significant (p-value < 0.05).
The distance spinal dura mater and the spinal cord was corrected by angle (Table 3). The Friedman test suggests that there is a significant difference, returning a p-value close to zero. The following plot indicates which differences are significant (p-value < 0.05).
The distance measurement corrected by angle Spinal Dura Mater and the Spinal Cord shows being the 5th thoracic one (8.18±2.61 mm) is greater than both lengths of the 2nd (6.07±1.99 mm) and 10th (6.57±3.97 mm) (Wilcoxon test p-values < 0.05), and no evidence of difference amongst these two (Wilcoxon test p-values = 0.53).
Dura Spinal Cord: There is no significant difference between groups (p-values 0.81, 0.46, 0.39, respectively for T2, T5, T10). Skin: There is a significant difference between the groups (p-values 0.000000065, 0.0000000028, 0.0000000071, respectively for T2, T5, T10). Intervertebral Space: There is a significant difference between groups for T5-T6 and T9-T10 (p-values 0.55, 0.018, 0.046, respectively for T2-T3, T5-T6, T9-T10). Corrected: There is a significant difference between groups for T5 (p-values 0.52, 0.068, 0.95, respectively for T2, T5, T10).
This increase was observed both in children from 0 to 6 years old, as well as from 7 to 13 years old (Table 4, Table 5).
|
Variable (0 – 13 Years) |
2nd Thoracic |
5th Thoracic |
10th Thoracic |
||||||
|
min. |
mean |
max. |
min. |
mean |
max. |
min. |
mean |
max. |
|
|
SDM-SC |
1.7 |
4.52±1.25 |
8.3 |
2.3 |
5.61±1.60 |
10.2 |
2.2 |
4.68±1.38 |
8.2 |
|
Skin-SC |
16.7 |
33.79±8.22 |
60.9 |
16.7 |
32.13±8.58 |
59.6 |
8.4 |
28.96±8.97 |
58 |
|
Angle-Degree (o) |
15.6 |
27.16±5.12 |
44.9 |
21.7 |
38.68±6.10 |
56.2 |
23.3 |
39.08±5.23 |
53.3 |
|
DcAn-SDM-SC |
2.8 |
6.07±1.99 |
6.18 |
3.6 |
8.18±2.61 |
8.61 |
2.3 |
6.57±3.97 |
6.14 |
Table 3 Minimum, mean (standard deviation), and maximum, in millimeters, of spinal dura mater and spinal cord, skin to spinal cord distance, angle entry of needle, and distance corrected by angle in all 105 children
|
Variable (0 – 6 Years) |
2nd Thoracic |
5th Thoracic |
10th Thoracic |
||||||
|
min. |
mean |
max. |
min. |
mean |
max. |
min. |
mean |
max. |
|
|
SDM-SC |
1.7 |
4.44±1.23 |
6.9 |
2.3 |
5.42±1.54 |
8.5 |
2.2 |
4.54±1.34 |
7.7 |
|
Skin-SC |
16.7 |
29.51±6.29 |
60.9 |
16.7 |
27.39±6.87 |
59.6 |
8.4 |
24.22±7.23 |
57.7 |
|
Angle-Degree (o) |
15.6 |
27.10±5.64 |
44.9 |
26.5 |
37.33±5.16 |
56.2 |
23.3 |
38.04±5.09 |
53.3 |
|
DcAn-SDM-SC |
3 |
5.95±1.99 |
14.3 |
3.6 |
7.66±2.47 |
14.6 |
2.3 |
7.07±5.54 |
32.3 |
Table 4 Minimum, mean (standard deviation), and maximum, in millimeters, of spinal dura mater and spinal cord, skin to spinal cord distance, angle entry of needle, and distance corrected by angle in Group 1 (0 – 6 years)
|
Variable (7 – 13 Years) |
2nd Thoracic |
5th Thoracic |
10th Thoracic |
||||||
|
min. |
mean |
max. |
min. |
mean |
max. |
min. |
mean |
max. |
|
|
SDM-SC |
2 |
4.59±1.27 |
8.3 |
2.7 |
5.78±1.64 |
10.2 |
2.3 |
4.78±1.40 |
8.2 |
|
Skin-SC |
26.1 |
37.22±7.56 |
54.4 |
22.9 |
35.94±7.90 |
59.4 |
20.5 |
32.75±8.45 |
58 |
|
Angle-Degree (o) |
15.6 |
27.22±4.65 |
42.7 |
21.7 |
38.90±6.58 |
56.2 |
23.3 |
39.95±5.18 |
53.3 |
|
DcAn-SDM-SC |
2.8 |
6.17±1.99 |
12.9 |
3.901 |
8.65±2.66 |
14.8 |
2.9 |
6.12±1.76 |
10.1 |
Table 5 Minimum, mean (standard deviation), and maximum, in millimeters, of spinal dura mater and spinal cord, skin to spinal cord distance, angle entry of needle, and distance corrected by angle in Group 2 (7 – 13 years)
There was no correlation between gender and the insertion angle or needle trajectory length (Figure 4 - Figure 6).
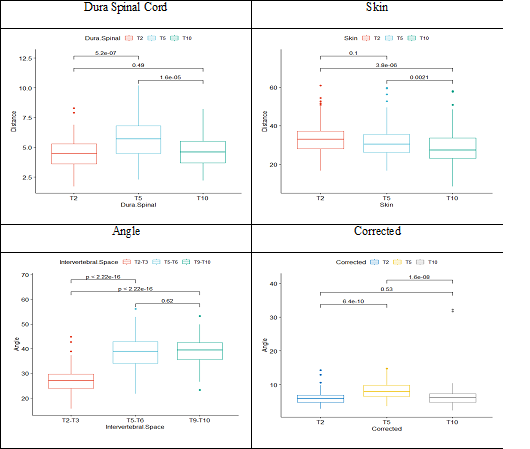
Figure 4 Comparison between the evaluated parameters and the correction by the obtained angle.
Dura Spinal Cord: There is no significant difference between groups (p-values 0.81, 0.46, 0.39, respectively for T2, T5, T10).
Skin: There is a significant difference between the groups (p-values 0.000000065, 0.0000000028, 0.0000000071, respectively for T2, T5, T10).
Intervertebral Space: There is a significant difference between groups for T5-T6 and T9-T10 (p-values 0.55, 0.018, 0.046, respectively for T2-T3, T5-T6, T9-T10).
Corrected: There is a significant difference between groups for T5 (p-values 0.52, 0.068, 0.95, respectively for T2, T5, T10).
MRI is the most significant technological advancement in the diagnostic examination of the spine. This study in 105 children aged 0 to 13 years confirmed that there was a greater depth of the posterior subarachnoid space in the upper thoracic (T2= 4.52 mm), mid-thoracic (T5=5.61 mm) and lower thoracic (T10=4.68 mm), similar to those found in adults.8,9 The needle entry angle increased the distance between the dura mater and the spinal cord, providing anatomical protection to prevent the needle from touching the spinal cord. The needle entry angle (T2=27.16º; T5=38.68º; T10=39.08º) increased the distance between the dura mater and the spinal cord in the three thoracic segments studied (T2=6.07 mm; T5=8.18 mm; T10=6.57 mm), providing anatomical protection to prevent the needle from touching the spinal cord, during neuraxial anesthesia in this region. This increase in the distance between the dura mater and spinal cord was observed both in children from 0 to 6 years old, as well as from 7 to 13 years old, and ranged from 34% to 45%.
MRI can show all structures in various planes using different pulse sequences that allow optimal characterization of the tissues in and around the pediatric spine. CSF and fat act as natural contrast agents in spinal MRI. They provide excellent contrast for outlining the spinal cord, the thecal sac, and the nerve roots and veins within the neural foramina. Multiple substantial changes occur in the vertebral ossification center and the intervertebral discs that markedly alter the overall appearance of the spine, especially between infancy and 2 years of age.11 The evolution characterized by MRI of the pediatric spine between childhood and 2 years of age has been studied,11 and the dynamic process up to the age of 10 years has been well described.12 This study was carried out with children from 0 to 13 years old, who were later divided into two groups and the results did not differ from the different distances evaluated between the two groups.
The spinal cord is the segment of the central nervous system that lies within the spinal canal caudal to the foramen magnum. The spinal cord is a tubular neural structure situated with the cranial two-thirds of the spinal canal.13 The anatomy of the human being allowed for a space between the dura mater and the spinal cord, allowing for protection in case of entry of a needle both in spinal anesthesia and in accidental perforation of the dura mater during epidural thoracic, distance confirmed in horizontal measurement (4.51 to 5.61 mm), increased to (6.07 to 8.18 mm) corrected for the needle entry angle, in this study with children aged 0 to 13 years.
Studying 19 adult patients with an MRI scanner while recumbent in the supine position, showed that there is a greater depth of the posterior subarachnoid space at mid-thoracic levels than at lumbar and upper thoracic levels.14 This fact was confirmed in 50 adult patients.9 This indicates that potentially there is more space available for intrathecal delivery of anesthetic drugs while avoiding spinal cord contact in the thoracic region, a fact confirmed by performing thoracic spinal anesthesia in 1,406 adult patients.15 As there is this same space in the 105 children in this study, it will be possible to perform puncture (spinal or epidural anesthesia) at the thoracic level safely and without neurological damage.
There are significant anatomical differences in children compared with adults that should be considered when using neuraxial anesthesia, mainly in the final thoracic and lumbar regions. In neonates and infants, the conus medullaris ends at approximately the L3 level as opposed to adults, where it is located approximately at the L1 vertebra. At approximately 1 year of age, the conus medullaris reaches the L1 level, similar to that in an adult. Imaging studies have shown that in children 2 to 10 yr of age, the mean distance of the spinal cord from the dura mater at the T9-T10 vertebral level is 4.3 mm.16 In our study, the values found in the three thoracic segments were similar to those obtained for 4.3 mm, and no significant difference was observed between the two groups (0-6 vs 7-13 years) and was highest at the T5 level.
The objective of studying the distance from the skin to the epidural space was performed with MRI studies at multiple levels, in 108 children focusing on T6‑T7, T9‑T10, and L2‑L3.17 The average of the results obtained from the distances in the three spaces was: 18.2 mm and 21.6 mm at T6‑T7; 18.3 mm and 20.5 mm at T9‑T10; and 21.8 mm at L2‑L3, concluding this distance is not constant at various vertebral levels. In our study, the distance between the skin and the epidural space was not measured, but the distance between the skin and spinal cord obtained slightly larger distances (T2=33.79 mm; T5=32.13 mm; T10=28.96 mm). This result confirms that the spinal cord has a greater depth than the epidural space.
The distance from the dura mater to the spinal cord is not uniform at different vertebral levels. Using MRI to measure the distance between the dura mater and spinal cord in two thoracic levels T6-T7, and T9-T10, and one lumbar level L1-L2 using MRI images, performed in 88 children under the age of 8 years old.18 The results obtained were 5.9±1.6 mm at T6-T7, 5.0±1.6 mm at T9-T10, and 3.6±1.2 mm at L1-L2. The largest dura mater to spinal cord distance is found at the T6-T7 level and the shortest dura mater to spinal cord distance is at the L1-L2 level.18 Similar results to those found in the study with children aged 0 to 13 years, with the greatest distance at T5 (5.61 mm) being slightly smaller than 5.9 mm. This distance increased to 8.18 mm corrected for the angle of a possible spinal needle entry.
The Quincke needle tip is already the entry of the CSF, which should be 1.25 mm.15 As for the Whitacre needle, its lateral orifice ends with a distance of 2 mm.15 Both of these distances are much smaller than the distance between the dura mater and the spinal cord.
The mean needle entry angle found in adults was 9º at T2, 45º at T5, and 9.5º at T10.8 The mean of the angle found in children aged 0 to 13 was 27.16º at T2, 38.68º at T5, and 39.08º at T10, reflecting a greater distance between the dura mater and the spinal cord. The result of this study with children from 0 to 13 years old, showed a different curvature from the adult, with the angle at T9-T10, greater than that obtained in adults.8
Knowing that there is a significant difference in the distance between the dura mater and spinal cord at different thoracic levels, it provides valuable information for choosing the site where the thoracic puncture for spinal anesthesia will be performed15 and also the use of a catheter in continuous thoracic spinal anesthesia providing a greater margin of safety.19
Accidental dural puncture in adult patients during needle insertion occurred in 0.4% - 1.2% of instances in a series of 6,496 thoracic epidural blocks, but none of the 48 patients developed subsequent neurologic sequelae.20-22 In four cases (2 lumbar punctures and 2 thoracic punctures) illustrate severe permanent or long-standing neurological injury after uncomplicated placement of epidural catheters in children.24 In each of these cases, the neurological result was devastating, and 3 cases resulted in sizeable medical malpractice claims and settlements despite the absence of proof of medical negligence.
Recently MRI was used and represents a non-invasive alternative to a full autopsy in neonatal death if parents refuse a classical full autopsy.24
The lumbar spine is the most frequently imaged region in both children and adults. MRI is an excellent modality for imaging the pediatric spine. A normal MRI study was defined as one that demonstrated a normal medullary cone located above the intervertebral space between the second and third lumbar vertebra. Appreciating normal MRI anatomy is essential for understanding and measuring the different distances proposed in this study. However, there are few studies with MRI in the thoracic region of children. This study in children demonstrated there is a greater depth of the posterior subarachnoid space at T2, T5, and T10 levels. The greater distance was found at T5. The correction of the needle’s entry angle increased the distance in the three interspaces. Because of the risk of a medullary lesion caused by needle-tip injury, the distance between the dura mater and the cord is of great importance. This distance between the dura mater and the spinal cord protects the spinal from damage in case of an accidental dural puncture during thoracic epidural attempts. It certainly will permit a spinal needle to provide segmental thoracic spinal anesthesia without damage in children.
None.
Authors declare that there is no conflict of interest.

©2023 Imbelloni, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.