Journal of
eISSN: 2373-633X


Review Article Volume 2 Issue 6
Department of Health Informatics, Brooks Rehabilitation College of Health Science, Jacksonville University, USA
Correspondence: Patrick Casimir PhD, Program Director and Assistant Professor Health Informatics, Brooks Rehabilitation College of Health Sciences, Jacksonville University, Jacksonville, FL 32211, USA, Tel 904-256-8917
Received: April 27, 2015 | Published: July 9, 2015
Citation: Casimir P. Study review on the role of image analysis on improving diagnostic and therapeutic breast cancer outcomes. J Cancer Prev Curr Res. 2015;2(6):179-183. DOI: 10.15406/jcpcr.2015.02.00060
This paper examines the critical application of image analysis in improving the diagnosis and management of breast cancer using techniques such as mammography, dedicated breast computed tomography (DBCT), background parenchymal enhancement (BPE), positron emission tomography, optical imaging, molecular imaging, automated whole breast ultrasound (AWBU), mechanical imaging, and optical coherence tomography (OCT). The paper performs a review of previously published studies and research on various imaging techniques currently used in the overall screening and assessment of breasts’ size, volume, composition, and density. Using findings from those studies, the paper demonstrates that such methods have been successfully applied into clinical practice to ameliorate health conditions of breast cancer patients and reduce mortality rate.
Keywords: breast cancer, image analysis, mechanical imaging, dedicated breast ct (dbct), background enhancement, mammographic screening, positron emission tomography, percentage mammographic density (PMD), microscopic observations, mammographic breast composition, magnetic nano-particles, ultra-sensitive magnetic field sensors, mortality rate
DBCT, dedicated breast computed tomography; PMD, percentage mammographic density; BPE, background parenchymal enhancement; AWBU: automated whole breast ultrasound; OCT, optical coherence tomography
The healthcare industry has been making significant changes to the way healthcare services are delivered to the American public. Such important changes are the increasing enhancements of technologies such as image analysis within healthcare facilities across the country to better diagnose and manage diseases such as breast cancer. Accordingly, Gonzalez & Woods,1 in their book Digital Image Processing, define image analysis as a “process of discovering, identifying, and understanding patterns that are relevant to the performance of an image-based task. One of the principal goals of image analysis by computer is to endow a machine with the capability to approximate, in some sense, a similar capability in human beings.”1 In the same context, Baxes G2 depicts image analysis as operations that are used to produce the measurement and classification of image information by the quantification of elements such as cells, bolts, and characters (p. 123). Thus, this paper represents an overview of multiple clinical studies of major image analysis applications such as mammography, dedicated breast computed tomography (DBCT), background parenchymal enhancement (BPE), positron emission tomography, optical imaging, molecular imaging, automated whole breast ultrasound (AWBU), mechanical imaging, and optical coherence tomography (OCT) to show their significant usage in improving breast cancer screening as well as diagnostic and therapeutic outcomes.
Background
The application of image analysis in detecting and managing breast cancer is far from being a new pursuit. According to Van Steen & Van Tiggelen,3 the first attempts to use radiography for the screening of breast irregularities began as far back as in 1913 with the work of A. Salomon “in the confrontation of this first time semiology of macroscopic anatomy with microscopic examinations”, yet modern mammography with dedicated x-ray units did not start until 1960s. From the 1960s until now, image analysis within the field of medicine has increasingly evolved and improved as to make it possible that “computer procedures enhance the contrast or code the intensity levels into color for easier interpretation of x-rays and other biomedical images.”1 Thereby, Baxes G agrees and writes “From the 1960s through today, the evolution of the digital computer has certainly been largely2 responsible for enabling the proliferation of digital image processing applications. Costly mainframe computers are no longer a requirement of the digital image processing equation like they were in the 1960s. The advent of microprocessors, leading to the personal computer, has allowed stand-alone digital image processing applications to become available.” (p. 7).
The use of mammography imaging within healthcare practice is universally accepted as a critical tool to help improve the detection and screening of breast cancer with the emphasis for better diagnosis, treatment, management, and reduction in breast cancer mortality in general. Notably, this pressing need has led to numerous studies on the impact of mammography as an imaging technique that uses x-ray units to analyze, detect, and evaluate changes in breast mass and composition. Hence, in the study Increasingly Strong Reduction in Breast Cancer Mortality Due to Screening, Schoor VG et al.,4 conduct mammographic screenings of 55,529 women and compare death rate breast cancer among screened versus unscreened women. Using that study, Schoor VG et al.,4 show that mammographic exams among women can lead to an overall reduction of 35% in breast cancer mortality. In consequence, as shown in Table 1 listed below, they concur “The breast cancer death rate in the screened group over the complete period was 35% lower than in the unscreened group (OR=0.65, 95% CI=0.49–0.87). Analysis by calendar year showed an increasing effectiveness from a 28% reduction in breast cancer mortality in the period 1975–1991 (OR=0.72, 95% CI=0.47–1.09) to 65% in the period 1992–2008 (OR=0.35, 95% CI=0.19–0.64).”4
The Effectiveness of Mammographic Screening on Breast Cancer Mortality Expressed by Odds Ratios, According to Calendar Period of Index-Invitation at Screening and Corrected for Age at Invitation |
|||
Calendar Period of Index-Invitation |
Cases Screened (unscreened) |
Referents Screened (unscreened) |
Odds Ratio (95% Cl) |
1975-2008 |
191(91) |
1089(321) |
0.65(0.49-0.87) |
1975-1991 |
90(40) |
501(149) |
0.72(0.47-1.09) |
1992-2008 |
29(23) |
202(58) |
0.35(0.19-0.64) |
Table 1 Comparison of breast cancer mortality Odds Ratios among women
CI, confidence interval
Furthermore, in another study An X-ray Computed Tomography/Positron Emission Tomography System Designed Specifically for Breast Imaging Statistical analysis of mammographic breast composition measurements: towards a quantitative measure of relative breast cancer risk, Kotre CJ5 uses mammographic devices with x-ray absorptiometry to investigate “glandular thickness” in women’ breasts. Findings from Kotre CJ5 study show that it is possible to determine the risk of breast cancer among women using mammographic imaging to measure breast composition as show in Figure 1 below. Kotre CJ5 consents that “It is demonstrated that a non-linear transformation can be used to produce normal statistical distributions, suitable for producing a standardized “Z-score” for breast composition. A standard “Z-score” approach to identify women with unusually glandular breasts is recommended and so provide a basis for cancer risk estimations”. Earlier we have shown that mammography can be valuable in conducting breast cancer screenings and reducing mortality among screened patients. Nonetheless mammographic operations present some critical limitations. Accordingly, Lindfors KK et al.,6 explain “One inherent limitation of planar mammography is that a three-dimensional, volumetric structure (the breast) is imaged, but the resultant data are displayed in a two- dimensional manner. This leads to superimposition of tissue and limits effectiveness, especially in women with dense breasts.” Studies have demonstrated that computed tomography can be helpful in alleviating those limitations and providing a higher sensitivity in cancer detection than mammography. In their study, An X-ray Computed Tomography/Positron Emission Tomography System Designed Specifically for Breast Imaging, Boone et al.,2 show that computed tomography of the breast, as shown below in Figure 2, as an imaging technique produces higher resolution images with the potential of improving breast cancer detection through screening. In that context, they write “Because the breast CT data set is coherent, meaning it is a true 3D volume data set acquired of the entire breast in one position, the axial (x, z), sagittal (y, z) and coronal images (x, y) have a well-defined spatial relationship between each other. This allows the observer to click on an area of suspicion in one view, and the viewer then instantly presents the other two orthogonal views which intersect that same point of suspicion. This feature allows the observer to rapidly search the 3D volume data set in an interpretive mode. The rapidity of the visualization tool working with the GPU hardware, combined with the display features mentioned above, allow the radiologist observer to comprehend the image data not as separate 2D images as with mammography, but rather as a coherent 3D volume of image data”. In that same study, Boone JM et al.,7 exhibit a graph, shown in Figure 3, that computes breast lesion size against sensitivity @ 95% specificity for mammography compared to computed tomography to reveal that computed tomography of the breast is a much better tool in detecting mass lesion in breast and screening of breast cancer. Moreover Lindfors KK et al.,6 in the study Dedicated Breast CT: The Optimal Cross Sectional Imaging Solution?, recognize the superiority of computed tomography in improving the discovery of malignant lesions in women’ breasts and write: “Three- dimensional visualization of the breast mitigates the limiting effects of superimposition noted with mammography. Post-processing capabilities will allow application of advanced technologies, such as creation of MIP and subtraction images, as well as the use of both computer-aided detection and possible computer-aided diagnosis algorithms. Excellent morphologic detail and soft tissue contrast can be achieved, due in part to the isotropic image data that DBCT produces”. Throughout the study, they show why dedicated breast computed tomography (DBCT) has the capabilities to reduce the overall number of patient recalls and increase sensitivity for masses. Also, they endorse the use of dedicated breast computed tomography (DBCT) in corroborating previously detected findings of lesions or masses through mammographic screenings.
Another imaging analysis technique that has been found to produce very accurate results in terms of assessing breast cancer risk is magnetic resonance imaging (MRI), which is a significant apparatus for better diagnosis, treatment, and management of breast cancer. As a technique, MRI generates images of internal structures of the human body by using magnetic fields and pulses of radio energy. Many studies have shown that MRI is a better and more accurate tool to be used in the detection of breast masses and breast cancer in general because, as written by Wasif N et al.,8 “MRI is more accurate than either ultrasonography or mammography for assessment of the size of primary breast cancer presenting as a mass”. In fact, in Growth of Breast Cancer recurrences Assessed by Consecutive MRI, a recent study conducted by Millet I et al.,9 the application of MRI as an image analysis technique to perform comparisons between the increase of primary breast cancer and reappearances in women who have previously had a MRI. In that aspect, Millet et al.,9 design the following methods: “All participants underwent dynamic, contrast-enhanced breast MRI. Minimum standard criteria were required for each MRI study performed: a 1.5-T magnet, a dedicated breast-surface coil, and one image obtained before and dynamic images obtained after the administration of contrast material, with three-dimensional, T1-weighted, gradient-echo sequences. Spatial-resolution criteria included voxels smaller than 0.7 mm in the frequency-encoding direction, smaller than 1 mm in the phase-encoding direction, and 3 mm or smaller in the slice direction, thus providing full coverage of the breast”. Thus, Millet et al.,9 conclude that women with a previous diagnosis of breast cancer have a greater risk of “developing an ipsi- or contralateral recurrence” as proven in the diagram below of Figure 4.
Similarly, King et al.,10 conduct a study on background parenchymal enhancement (BPE), a MRI related technique, used to measure tissue enhancement in breast fibroglandular tissue regions on contrast-enhanced breast MRI images aimed at quantifying the enhancement of breast parenchyma as shown in Figure 5. In their study, 1275 women underwent breast MRI screenings and comparisons of normal controls and false controls findings were made to investigate “the level of MR imaging–depicted BPE and the amount of MR imaging–depicted FGT by using a categorical scale: BPE was categorized as minimal, mild, moderate, or marked, and FGT was categorized as fatty, scattered, heterogeneously dense, or dense”.10 According to King V et al.,10 odds ratio for breast cancer are increasingly higher for significantly greater BPE. Taken together, it was shown that high BPE is a predictive determinant of breast cancer risk and that the MR imaging analysis technique can be a useful tool in the diagnosis and reduction of breast cancer among larger populations of women.
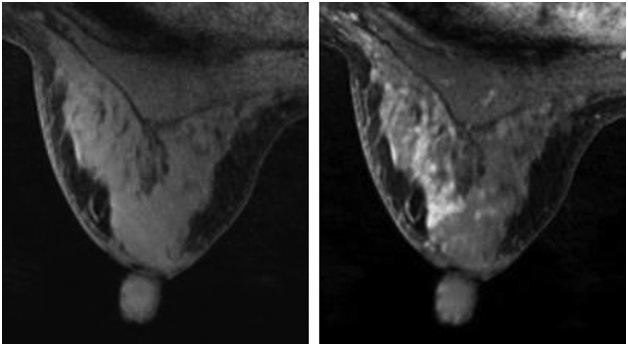
Figure 5 Background parenchymal enhancement of a woman breast.9
Although mammography is generally accepted as the standard method for breast cancer screening and detection since the 1960s, an array of studies have shown that too often mammography fails to detect numerous cancers. As noted earlier, new emerging techniques such as CT and MRI have proven to be significantly more accurate than mammography in breast cancer screening and more useful in helping diagnose and reduce breast cancer as a whole. Another imaging analysis technique deemed to provide improvements over mammography is automated whole breast ultrasound (AWBU), a process that uses an automated Xerox-like method to generate ultrasound images of breast tissue. AWBU can successfully be used in the screening of women with thick breast tissue, for whom conventional mammography is likely insufficient. Remarkably, in the study Breast Cancer Detection Using Automated Whole Breast Ultrasound and Mammography in Radiographically Dense Breasts, Kelly KM et al.,11 conduct mammographic screenings for 4419 women using mammography alone and compare those results to mammographic screenings with AWBU. Results of the study have shown that AWBU is drastically better than mammography at detecting breast cancer among women with high risk of breast cancer at a sensitivity ratio of 81% to 33% as demonstrated in Table 2. According to Kelly et al.,11 study, “Breast cancer detection doubled from 23 to 46 in 6,425 studies using AWBU with mammography, resulting in an increase in diagnostic yield from 3.6 per 1,000 with mammography alone to 7.2 per 1,000 by adding AWBU. PPV for biopsy based on mammography findings was 39.0% and for AWBU 38.4%. The number of detected invasive cancers 10 mm or less in size tripled from 7 to 21 when AWBU findings were added to mammography”. In the last decade, another imaging analysis technique that has been increasingly and effectively applied to breast cancer detection and diagnosis is mechanical imaging. Mechanical imaging is defined as “a branch of Elasticity Imaging, a medical diagnostic technique based on the visualization of tissue internal structures in terms of their elasticity”.12 In consequence,12 designed the study, Differentiation of Benign and Malignant Breast Lesions by Mechanical Imaging, in which they aimed to evaluate the effectiveness of mechanical imaging in detecting breast cancer through the differentiation between benign and malignant breast lesions. The study confirms the role of mechanical imaging as a critical technique that can be significant in enhancing the diagnosis of breast cancer and decreasing the rate of benign breast lesions. Also it proves that mechanical imaging can become a better alternative screening tool to mammographic screening of breast cancer. Largely, Egorov & Sarvazyan12 illustrate the critical performance of mechanical imaging in the detection of breast lesions as shown in Figure 5 and advance “The multisite clinical study proved the capability of mechanical imaging for real time characterization and differentiation of benign and malignant breast lesions. The BMI has the potential to be used as a cost effective device for cancer diagnostics, and it could effectively reduce the benign biopsy rate. The BMI has the potential to be positioned as an adjunct to mammography and utilized as a screening device for breast cancer detection” (Figure 6). So far in this paper, we have mentioned and reviewed several imaging analysis techniques that are well known and broadly used in the diagnosis and management of breast cancer. Interestingly there are few lesser used techniques that have provided considerable results in breast cancer detection. One such technique, positron emission tomography (PET) shown in Figure 7, creates images of organs within the human body by using a combinational process that includes a special camera and a radioactive chemical tester. Fascinatingly, in a 2010 study PET Tracers for Clinical Imaging of Breast Cancer, Penuelas I et al.,13 focus on how PET is currently utilized for reach higher sensitivity in the detection “of both lymph node infiltration and distant metastases compared to conventional imaging techniques”. Penuelas I et al.,13 offers statistical findings to confirm the results of their study in terms of potential improvements in the screening of breast tumors. In the same context, Avril N et al.,14 in the study Breast Imaging with Positron Emission Tomography and Fluorine-18 Fluorodeoxyglucose: Use and Limitations describe the image analysis process in PET. They write: Attenuation corrected images were reconstructed and normalized for injected dose and body weight resulting in parametric images representing regional standardized uptake values (SUV).24 SUV normalized images were printed on x-ray film using a linear gray scale. SUV values ranged between zero and five using a background threshold correction of 5%. PET images were visually analyzed by two observers blinded to clinical history or prior examinations. Regional FDG uptake in breast tissue was visually classified in three categories: PET scans with regional FDG uptake within the background activity of normal breast tissue were considered grade 1 (unlikely), PET scans with diffuse or moderate focally increased FDG uptake were considered grade 2 (probable), and cases with focally marked increased FDG uptake were considered grade 3 (definite) to represent malignant tissue. A consensus interpretation was reached for each patient. Statistical analysis was calculated two ways for visual image interpretation. Conventional image reading (CIR) was obtained by regarding only focal tracer accumulation (grade 3) as to represent malignancy, and sensitive image reading (SIR) was achieved by including probable (grade 2) and definite (grade 3) malignant lesions. In addition to positron emission tomography (PET), optical imaging is another lesser known imaging analysis method that has been agreed upon as an emerging technique with the potential to allow significant improvements in the diagnosis of breast cancer. As a technique, optical imaging uses light to probe tissue composition and biomolecular processes inside the human body. The practical process, as seen in Figure 8, consists of the application of infrared-light in assessing optical features of internal tissues and other molecular functions to create images that can be used in the biomedical field for better diagnosis of diseases such as breast cancer. Recent studies have been conducted to determine possible ways to enhance breast cancer diagnosis and treatment using optical imaging. One such study, Optical Imaging in Breast Cancer Diagnosis: The Next Evolution written by Herranz M & Ruibal A,15 discusses the medical application of optical imaging in the screening and diagnosis of breast cancer. Also in the study, Herranz & Ruibal15 analyze critical aspects and fundamentals of optical imaging that have favored the ongoing emergence and development of this imaging analysis technique as a valuable alternative to mammographic screenings and other imaging modalities used to detect and deter breast cancer. In all, Herranz & Ruibal15 suggest that optical imaging can help detect breast cancer earlier than other techniques and write: “Until now, studies have focused on using the intrinsic optical properties of the breast to visualize lesions without the use of fluorescent contrast agents. These studies described higher absorption for carcinomas than for the surrounding parenchyma due to increased blood content associated with angiogenesis. However, intrinsic contrast alone is probably not sensitive enough for (early) lesion detection. Optical breast imaging using a fluorescent contrast agent may improve lesion contrast and can potentially detect changes in breast tissue earlier. The fluorescent probes can either binds specifically to certain targets associated with cancer or can nonspecifically accumulate at the tumor site, mostly by extravasations through leaky vessels”. Finally, an imaging analysis method that was also found to be helpful in the diagnosis and management of breast tumors is optical coherence tomography (OCT). In the study Optical Coherence Tomography: The Intraoperative Assessment of Lymph Nodes in Breast Cancer, Nguyen FT et al.,16 define OCT as: OCT is a high-resolution microscopic optical imaging technique that yields real-time multidimensional images of subsurface tissue structure. The use of NIR light enables micron-scale resolution, providing structural images on a resolution scale similar to histopathology. The penetration depth in tissue has been found to be 1–2 mm and is highly dependent on the type of tissue imaged. OCT has been investigated in a large number of clinical applications ranging from ophthalmology, cardiovascular disease, Barrett's esophagus, and more recently in oncology. OCT has also been used to image tumor margins for breast cancer in an NMU carcinogen-induced rat mammary model and intraoperatively for the assessment of tumor margins. High-scattering signals are often attributed to a combination of the increase in nuclear-to-cytoplasm (N/C) ratio and the increase in cellular density during the focal proliferation of cancer cells. OCT is a suitable imaging modality for assessing the lymph node architecture but, more importantly, for imaging and assessing the morphological changes observed in the cortex, which can be used to differentiate between normal nodes and reactive and metastatic nodes. By imaging through the capsule of an intact lymph node, OCT can provide this assessment without compromising the structural integrity of the lymph node, making it a potential candidate as an in vivo nodal-assessment technique.17
Imaging Method/s |
Number of Cancers |
Number of Biopsies |
Percentage Positive |
95% Cl |
All positive mammograms |
23 |
59 |
39.00% |
26.6 to 52.6% |
All positive AWBU |
38 |
99 |
38.40% |
28.8 to 48.7% |
AWBU positive, mammogram negative |
23 |
75 |
30.70% |
20.5 to 42.4% |
Mammography and AWBU positive |
15 |
24 |
62.50% |
40.6 to 81.2% |
AWBU negative, mammogram positive |
8 |
35 |
22.90% |
10.4% to 40.1% |
Table 2 Positive predictive value of biopsy recommendation by imaging methods
AWBU, automated whole breast ultrasound; CI, confidence intervals
In conclusion, based on what has been demonstrated earlier in this paper, it is obvious that numerous imaging analysis techniques such as mammography, dedicated breast computed tomography (DBCT), background parenchymal enhancement (BPE), positron emission tomography, optical imaging, molecular imaging, automated whole breast ultrasound (AWBU), mechanical imaging, and optical coherence tomography (OCT) have been recognized and agreed upon by many writers and experts as a useful means for improving and aiding in the screening, diagnosis, and treatment of breast cancer. Remarkably, results and findings of various studies previously cited in this paper, have clearly corroborated the critical use of imaging analysis in ameliorating breast cancer outcomes and the pressing need to further develop and apply new emerging image analysis techniques to breast cancer detection, treatment, and research.
None.
The authors have no financial conflicts of interest to declare.
None.

©2015 Casimir. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.