Journal of
eISSN: 2373-633X


Research Article Volume 5 Issue 1
1Department of Clinical Oncology, Bahrain
2Department of Clinical Oncology, Dubai hospital, UAE
3Department of Clinical Oncology, Egypt Air hospital, Egypt
Correspondence: Dina Hamza, MSc Clinical Oncology, Medical Surveys-17 Research Group, scientific coordinator, Senior Medical Oncologist, Dubai hospital, Dubai
Received: April 26, 2016 | Published: May 27, 2016
Citation: Aref A, Hamza D, Fayed R. Screening of clinical practice in management of progression on trastuzumabi metastatic breast cancer. J Cancer Prev Curr Res. 2016;5(1):202-204. DOI: 10.15406/jcpcr.2016.05.00147
Background: Guidelines regarding treatment post progression on Trastuzumab have recently varied with lots of options available. Decision whether to continue on Trastuzumab or to switch to other anti-Her2 treatment varies due to many factors, including the guidelines recommendations, definition of resistance to Trastuzumab, availability of drugs, and socioeconomic factors. The objective of our survey is to evaluate and asses these factors.
Material and methods: The survey was emailed to more than 600 oncologists worldwide through BGICS database. Three main questions were covered by the survey; Definition of progression on Trastuzumab, how to deal with progression, and the factors affecting decision making.
Results: The survey was send to 612 oncologists worldwide, 167 replied. Participants were mainly from Egypt, Romania, and France. 28.7% consider resistance to Trastuzumab if progression happened while on Trastuzumab, 28.7 % consider resistance if any progression happened after receiving Trastuzumab whatever the duration or the site of progression, 18% consider resistance if progression took place after less than 6 months of receiving Trastuzumab. In case of CNS progression 35.3% had chosen start on Lapatinib, while 25.1% had chosen to change the chemotherapy and continue on Trastuzumab. 18.6% had chosen to give combination of Lapatinib with Trastuzumab.
Conclusion: This is the second publication of Medicalsurveys-17 Research Group. Our data revealed that more effort is required to define resistance to Trastuzumab and how to manage patients based on type of resistance and site of progression.
Mini abstract: Through this survey, we tried to define the oncologist perspective about resistance to Trastuzumab, the approach to deal with this progression and the factors that affect decision making.
Keywords: trastuzumab, progression on trastuzumab, resistance to trastuzumab, breast cancer
The HER2 oncogene encodes one of four transmembrane receptors within the erbB family. It is an over-expression which occurs in approximately 25% of all breast cancer tumours.1 Human epidermal growth factor receptor HER2 over-expression is associated with a shortened disease-free interval and poor survival.2 The addition of trastuzumab to chemotherapy in the first-line setting has improved response rates, progression-free survival, and overall survival. Meanwhile the response rates declined when trastuzumab was used beyond the first-line setting. In the recent years, many options had evolved in treatment after progression on Trastuzumab, even though the best treatment remains unclear. The definition of resistance to Trastuzumab as well as the site of progression (mainly CNS progression versus non CNS progression) still represents a point of debate between oncologists.
Recently the guidelines regarding the options of treatment after progression on Trastuzumab have changed. In the NCCN guidelines (V1 2013) using Pertuzumab with Trastuzumab is now the preferred option of treatment.3 The decision of treatment beyond progression on Trastuzumab differs between oncologists. Factors that affect this decision are mainly the presence of clear guidelines, the availability of the drugs as well as its cost and finally the physician clinical experience.
This survey represents the second work for “MedicalSurveys-17” Research Group. This group is based in Egypt and was created by the cooperation between groups of oncologists in the Middle East. This survey was done in collaboration with the BGICS. A web page was created which asked the oncologist about 3 main points after collecting some demographic and general information about their practice. The three main points which are covered by this survey were; definition of progression on Trastuzumab, second how oncologists deal with progression on Trastuzumab in ideal situation and in real practice and the last question is what are the factors that mainly affect the decision of treatment.
This survey was sent to about 600 oncologists worldwide using the BGICS data base as well as our group “Medicalsurveys-17” data base. SPSS software was used to analyze and prepare the descriptive data.
The Results of this project was presented as a poster and as an oral presentation during the 6th BGICC that was held in Cairo, Egypt in January 2014.
The survey was sent to 612 oncologist world wide, from which 167 have replied. 31.7% of the responders work in university hospital while 24 % work in governmental institute and 22.2% in the private sector. 55.7% has mentioned that treatment in their institute is covered by the government. Participants were mainly from Egypt, Romania, France, India, Kingdom of Saudi Arabia, and Italy.
The availability of the treatment options in this setting was assessed. In response to the question “what are the available drugs in your center?” 95.8% have Trastuzumab available in their center, while 76.6% have Lapatinib available. Only 11.4% have Pertuzumab and 6% have T-DM1. 92.8% were using Trastuzumab in both the adjuvant and metastatic setting. In response to one of the main points of this survey which is definition of resistance to Trastuzumab, 28.7% consider resistance to Trastuzumab if progression happened while on Trastuzumab. Also 28.7 % consider resistance if any progression happened after receiving Trastuzumab whatever the duration or the site of progression. 18% consider resistance to Trastuzumab if progression took place after less than 6 months of receiving Trastuzumab. 15% consider resistances if progression took place at any site other than CNS (mainly brain) (Figure 1).
We have asked a question regarding the management of progression in the form of CNS metastasis as only site of progression, and when giving the participant the options that he\she had all the facilities available, 35.3% had chosen to change chemotherapy and start on Lapatinib, while 25.1% had chosen to change the chemotherapy but to continue on Trastuzumab. 18.6% had chosen to give combination of Lapatinib with Trastuzumab. While only 6% chosen to start with Pertuzumab and Trastuzumab (Figure 2).
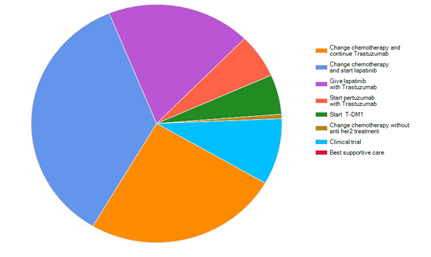
Figure 2 For patient with Her2 positive cancer. Who developed brain metastasis after treatment with transtuzumab, what types of treatment will you use. If you have all facilities available, after giving patient whole cranial irradiation.
When the same question was asked but in the form of Real Practice, still starting with Lapatinib and change of chemotherapy got the higher percentage, 44.3%, while continuation of Trastuzumab with changing the chemotherapy got 30.5%. Combination of both Trastuzumab and Lapatinib was chosen by 9% while only 1.8% had chosen to give Pertuzumab with Trastuzumab (Figure 3).
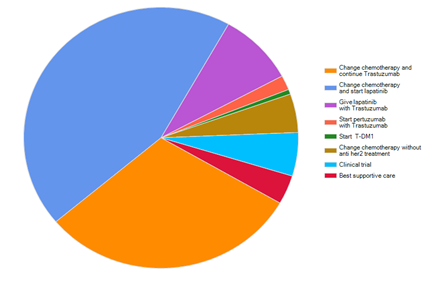
Figure 3 For the same previous question, what treatment do you really offer in your real daily practice.
In contrary to the previous case scenario, when assuming that all the facilities are available, the first choice for patient who developed visceral metastasis other than CNS was continuation on Trastuzumab and changing the chemotherapy, 31.7%, while 26.9% had chosen to start Lapatinib and change the chemotherapy. 16.8% had chosen starting T-DM1 and 10.8% had chosen to start Pertuzumab with Trastuzumab (Figure 4).
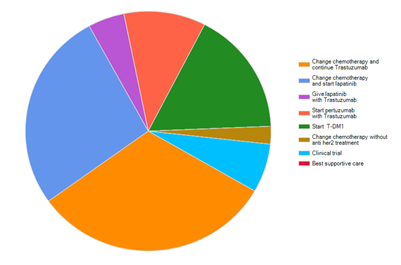
Figure 4 For patient with her2 positive breast cancer, who developed visceral metastasis other than CNS, after treatment with Transtuzumab what type of treatment will you use if you have all facilities available.
When the previous question was asked in consideration to what really happen in real practice, 38.3% had chosen to change the chemotherapy and start Lapatinib, while 36.5% had chosen to change chemotherapy but continue on Trastuzuamb. 9.6% had chosen to change the chemotherapy without anti Her2 treatment (Figure 5).

Figure 5 For the same previous question what type of treatment do you really offer in your real daily practice.
Regarding the third point of interest in our survey, which is the main factor affecting treatment decision? 32.3% stated that the availability of the drug is the main factor, while 28.7% had chosen the patient criteria as the main factor. 20.4% saw that the cost of the treatment is the main factor affecting their decision making while 18.6% had chosen the institute guidelines as the main factor (Figure 6).
The definition of resistance to Trastuzumab is still an open point for discussion. Timing of progression and the duration since last cycle of Trastuzumab as well as the site of progression are the main points of debate that are covered in this survey. Primary resistance to Trastuzumab occurs when it is ineffective for the treatment despite tumor expression of HER2. Secondary resistance occurs when patients who initially respond to Trastuzumab experience Trastuzumab-refractory relapse.4 Although this definition seems simple, it does not answer all the questions about early and late resistance and whether the approach for each scenario should be different. The data we got from the survey indicate that this point represent a point of confusion within oncologist and more work and more trials seems to be needed to clearly define resistance to Trastuzumab as well as specify the perfect approach in management.
Trastuzumab has impaired ability to penetrate the blood-brain barrier which represents an obstacle in preventing or treating brain metastases.5 Patients treated with Trastuzumab after brain metastasis was diagnosed experienced a longer survival compared with patients who received other chemotherapy. This survival result is consistent with those of similar studies that reported survival benefits with Trastuzumab therapy in patients with brain metastases.6
Lapatinib has been shown to reduce the formation of brain metastases in animal models. A phase II study of Lapatinib mono-therapy in 241 patients with progressive brain metastases after Trastuzumab and radiotherapy demonstrated a disappointing response rate of 2.6%, however the authors noted that 18% of patients were progression-free at 16 weeks with favorable volumetric changes in CNS disease.7
Our results present this controversy in defining whether CNS failure represents failure on Trastuzumab or no, and what the best treatment option to start with. Although changing chemotherapy and start with Lapatinib was the first choice, but the difference between this choice and continuation on Trastuzumab was not big. On the other hand, failure on Trastuzumab in the form of visceral metastasis, other than CNS, showed more controversy, as the decision was almost equal between these two options.
One of our great interests is to what extent the socioeconomic factors could affect daily practice. And to what extent the international guidelines could be applied. From the results we have got, we can see that about 50% of the oncologist decision is affected by the cost and the availability of the drug, from which we can conclude that socioeconomic factors could prevent patients from receiving the standard of care.
This is the second publication of MedicalSurveys-17 Research group. In this survey we are trying to highlight the areas of controversy in the management of patients who progressed on Trastuzumab. From the data we have got , it seems that more effort is required to define resistance to Trastuzumab and how to manage patients based on each type of resistance. Also, the approach to patients who developed solitary site progression in the CNS needs more trials to detect the best practice. Socioeconomic factors are a major factor in affecting the physician decisions, rendering the indicated drugs unavailable for physicians’ prescription.
None.
None.

©2016 Aref, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.