Journal of
eISSN: 2373-633X


Case Report Volume 14 Issue 1
1General Surgery Department, Hospital da Baleia, Brazil
2Plastic Surgery Department, Hospital da Baleia, Brazil
3Department of Medicine, Centro Universitário de Belo Horizonte, Brazil
Correspondence: Pedro Henrique Faria Silva Trocoli-Couto, General Surgery Department, Hospital da Baleia, Santa Rita Durão Street, number 20, Sl 708, Funcionários, Belo Horizonte – Minas Gerais – Brazil, Tel +55 (31) 99745-2649
Received: January 13, 2023 | Published: January 23, 2023
Citation: Santos EC, Junior GLF, Trocoli-Couto PHFS, et al. Regression of a Merkel cell carcinoma in the hypothenar region: a case report. J Cancer Prev Curr Res. 2023;14(1):5-7. DOI: 10.15406/jcpcr.2023.14.00510
Merkel cell carcinoma (MCC) is a rare and aggressive cutaneous neuroendocrine neoplasm with a poor prognosis. It usually affects white-skinned old individuals in areas chronically exposed to solar radiation. MCC’s spontaneous regression is a mechanism not yet clearly understood. It is a known fact and a rare occurrence in MCC, after biopsy or incomplete resection has been performed. Considering the rarity of this type of occurrence, we will describe a clinical case treated at Hospital da Baleia, Belo Horizonte, State of Minas Gerais, Brazil. The report will address a male patient, 72 years old, leukodermic, with systemic arterial hypertension, type 2 diabetes mellitus and vitiligo. He was referred to the surgery department with a lesion in the right hypothenar region with an onset 4 months before admission, and presented with tumor regression after biopsy. Data was assembled through the analysis of the patient's medical record, after declared authorization, open interview with the patient at the time of follow-up appointments to the hospital's outpatient clinic, and analysis of photographic records of the injury before and after the instituted therapy.
Keywords: Merkel cell carcinoma, neuroendocrine neoplasia, spontaneous regression, polyomavirus, neuroendocrine markers
Merkel Cell Carcinoma (MCC) is a rare and aggressive skin neoplasm, originated from Merkel cells located in the basal layer of the epidermis, associated with local mechanoreceptors and with neuroendocrine function.1 Although rare, MCC is one of the most aggressive skin cancers, and its incidence is dramatically.2 The regression observed in Merkel Cell Carcinoma is a rare phenomenon, with less than 40 descriptions found in the literature.11
Several risk factors are observed in the pathogenesis of MCC, with Merkel Cell Polyomavirus (MCPyV) being related to the development of most cases of MCC. In addition to other factors such as advanced age, ultraviolet radiation and chronic immunosuppression (8% of patients with CCM hematological malignancy, solid organ transplantation or HIV/AIDS).1,3,4
Differential diagnosis includes small cell lung carcinoma, melanoma and lymphoma.5,6 Histopathological analysis and immunohistochemical panel are diagnostic.5
Regression of primary MCC lesion that occurs after biopsy or incomplete excision is not clearly understood.5
Excision with wide margins is the recommended treatment.5 Cells from the MCC can spread to locoregional lymph nodes. Thus, sentinel lymph node biopsy (BLNS) is necessary in some cases.5,6 When lymph node involvement is identified, node dissection of the nodal basin is recommended.5
Due to high recurrence, surveillance in the first 2 years after the initial diagnosis of MCC should be periodical.5,6
A male patient, 72 years old, leukodermic, hipertensive, diabetic and with vitiligo, was referred with a lesion in the right hypothenar region that appeared 4 months earlier. The lesion was a hardened, erythematous, painless and adhered nodule, with growth and subsequent ulceration (Figure 1A). An incisional biopsy was performed 2 months after the onset of the lesion. A neuroendocrine neoplasm suggestive of MCC was evidenced, consisting of clusters of small cells, with a basaloid aspect, sparse cytoplasm, hypercolored nuclei and granular chromatin, and with a high proliferative index, forming a solid mass. Immunohistochemical study showed strong positivity for markers AE1 + AE3, CK20 (in “dot” pattern), chromogranin A, Ki-67 and Synaptophysin, compatible with MCC.
In the first visit to our department, lesion regression was observed. According to the patient, the ulcerated lesion decreased spontaneously and progressively after the biopsy (Figure 1B). Physical examination showed an enlarged lymph node in the right axilla, with a non-hardened consistency. Right hand MRI showed a cutaneous/subcutaneous lesion on the ulnar surface of the hand, at the level of the V metacarpal, extending to the surface of hypothenar muscle fascia.
The lesion’s clear margin resection was indicated and the sentinel lymph node biopsy with radiouptake and methylene blue infiltration showed hyperuptake in right antecubital and axillary lymph nodes (Figure 2), which were resected (Figure 1D). After excision of the lesion with recommended macroscopic margins, a total skin graft was performed in the hypothenar region (Figure 1C). The anatomopathological study identified histological findings compatible with MCC with clear margins. The antecubital tissue presented no findings of neoplastic. The axillary lymph node was positive for neoplastic infiltration.
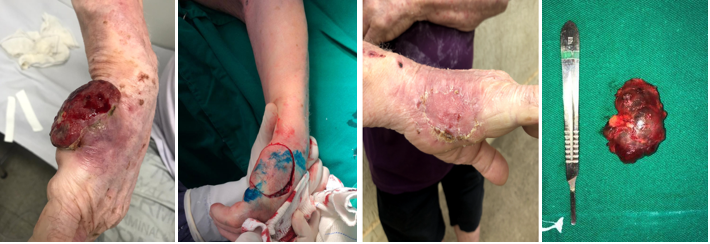
Figure 1 A, Initial lesion; B, Total regression of the lesion; C, Post-operative total skin graft in the hypothenar region; D, Axillary Sentinel Lymph Node.
Right axillary node dissection was performed. After analyzing the surgical specimen, 10 lymph nodes were isolated, without malignant findings. The patient returned for outpatient follow-up, and CT scans of the chest and abdomen and PET-CT were requested for staging and follow-up. Postoperatively, seroma formation in the right axillary region occurred, drained on an outpatient basis.
A relevant aspect in MCC pathology is tumor regression, a known phenomenon that occurs in some tumors, such as melanomas, but is rare in MCC with an estimated incidence betwen 1.5% - 3%.7,8 There are no known predictors of tumor regression, as its mechanism is not fully understood. The regression process is probably related to a T-cell immune reaction, linked to the activation of a response stimulated by surgical intervention and apoptosis.7 Histological findings showed an increase in immune activity around the tumor regression site in the form of infiltrating lymphocytes, in which the presence of foamy macrophages, fibrosis and CD3 +, CD4 + and CD8 + T lymphocytes were verified around the tumor nests. This reaction can be the result of surgical trauma caused by the biopsy or after incomplete excision of the primary tumor. In this sense, these findings raise the possible influence of the immune response mediated by T cells in the tumor regression process, leading to apoptosis and cell necrosis.7,8
There are reports, including metastatic MCC that presented regression. Studies have shown that stage III MCC with unknown primary site had a more favorable prognosis than those with an identified primary site, which demonstrates an important role in the immune mechanism regarding the evolution of the disease, even with nodal dissemination.5
Studies have shown that CCM is linked to Merkel Cell Polyomavirus (MCPyV). The virus is believed to induce tumorigenesis through the oncogenic action of T antigens. These cases have a better prognosis and longer tumor-free survival. This may be associated with the ability of the virus to stimulate the host's immune response.9
Up to 10% of MCC are diagnosed through nodal biopsies, with occult primary lesion, putting these cases in a better outcome when compared to those with cutaneous lesion with nodal dissemination.5 The prognosis is worse for patients with clinically affected lymph nodes, when compared to those with nodal dissemination identified on histopathological examination through sentinel lymph node biopsy (BLNS), fine needle aspiration puncture (FNAB) or node dissection.10 This observation is particularly important, since about 50-70% of patients progress for lymphatic dissemination.5,6,10 It is also important to report that 50 to 70% of patients with positive lymph nodes develop distant metastasis, with the most prevalent sites being the liver (13%), bones (10 - 15%), lungs (10-23%), brain (18%), skin at a distance (9-30%) and lymph nodes at a distance (9%).10 Relapses usually occur within 2 to 3 years of the initial diagnosis.5
The patient's immunohistochemical study showed positivity for AE1 + AE3, CK20 (in “dot” pattern), chromogranin A, Ki-67 and synaptophysin; and negativity for CDX2, CK7, PSMA and TTF1, compatible with MCC diagnosis. CK20 is the most prevalent cytokeratin in MCC, being absent in less than 10% of affected individuals.2,5,6 The neuroendocrine markers Synaptophysin and Chromogranin A show a strong relationship with MCC.2 MCC generally does not express thyroid transcription factor 1 (TTF1), ASH1, vimentin, S100B and CK7.5 Among these, CK7 and TTF1 were negative in the patient sample, corroborating the expected pattern.
The detection of Merkel cell papillomaviruses (MCPyV) can be a screening criterion for differentiating between MCC and other neuroendocrine tumors, since there is no association of this virus in neuroendocrine carcinomas of primary sites other than Merkel cells.5
Imaging exams for staging must be performed. Ultrasonography can be used to detect suspicious lymph nodes.5 PET-CT has proven to be the main technique, when compared to CT and MRI, as it achieves high rates of sensitivity and specificity in the detection of MCC metastases, since the Tumor cells have high metabolism, being able to detect even low tumor loads.2,5,6 A study demonstrated that PET-CT altered the staging of 33% of patients and directed a new therapy in 43% of cases.5
Surgery is the main treatment in a curative perspective.2 Excision with a wide margin of the lesion is recommended. The guidelines established by the "National Comprehensive Cancer Network (NCCN)" and "European Association of Dermato-Oncology “(EADO) - European Organization for Research and Treatment of Cancer" recommend a margin of 1–2 cm reaching, as a maximum limit, the muscular fascia or the pericranium. The enlarged margin aims to eliminate potential metastases by microsatellites.5
Head and neck regions may offer limitations to extended excision, with risks of functional loss and impaired quality of life.5,10 Thus, Mohs micrographic surgery can be performed.5
Lymph node involvement should be investigated.5 BLNS should be programmed concurrently with the resection of the primary lesion in patients without suspected lymph node involvement. Up to 30% of patients in clinical stages I or II have diagnosed nodal micrometastases and false negative results can reach 14.3%, with this higher rate in MCC located in the head and neck.5
With the diagnosis of lymph node involvement, drainage chain node dissection should be performed, as up to 30% of patients with subclinical nodal metastasis will harbor the disease in subsequent lymph nodes.5 For certain patients, wide-field radiotherapy at the site of the primary lesion is indicated, and in some cases, it is also indicated for lymph node basin drainage from the primary site as an adjuvant.5 This modality improves locoregional control and the rate of recurrence, but not overall survival.6 Radiotherapy can be used as an exclusive modality for patients who are not surgery candidates, as the MCC responds satisfactorily to this therapy, achieving disease control rates of up to 85%.5,6
Surveillance should be carried out in patients with MCC with a frequency of 3-6 months in the first 2 years and 6-12 months in the following years.5 Current guidelines recommend the use of imaging tests in the follow-up, especially of high-risk patients, which include disease with nodal or distant dissemination.5,6 As 80% of the recurrence rate occurs in the first two years after the initial diagnosis, patients who do not show signs of the disease after this period have a progressive reduction possibility of recurrence. After 5 years without relapses, follow-up can be carried out annually.5
Understanding the phenomena involved in the tumor regression of Merkel Cell Carcinoma will helps in identification and investigation of the disease, as well as in the treatment, prognosis and follow-up of patients, as these patients have an apparent better prognosis.
Sincere acknowledgments are expressed to the researchers responsible for writing this article, to the collaborators of the Hospital da Baleia, to the Centro Universitário de Belo Horizonte and to the patient with whom this case is treated.
The authors declare no conflicts of interest. Authors declare that the contents of this article are their own original unpublished findings.

©2023 Santos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.