Journal of
eISSN: 2373-633X


Research Article Volume 11 Issue 1
School of Science, Technology and Environment, Ana G. Mendez University (UAGM), USA
Correspondence: Beatriz Zayas, School of Science, Technology and Environment, Ana G. Mendez University (UAGM), Cupey, PO Box 21150 San Juan, Puerto Rico, 00928-1150, USA, Tel 787-766-1717
Received: January 10, 2020 | Published: January 23, 2020
Citation: Zayas B, Lebron V, Vélez C, et al. Novel NBQ-48 as marker of hypoxic cells in 2D and 3D colon cancer cells. J Cancer Prev Curr Res. 2020;11(1):13-18. DOI: 10.15406/jcpcr.2020.11.00417
This study presents the applicability of a novel nitro-substituted heterocyclic compound NBQ48, member of the family of compounds identified as 3 nitrobenzazolo[3,2-a]quinolinium chloride salts (NBQS) as a screening tool to identify hypoxic tumor cells. The applicability was tested on COLO 205 colon cancer cells 2D and 3D cultures treated with NBQ48 to assess the formation of a bio-reduction fluorescent metabolite under hypoxic conditions in contrast, to those under aerobic environment. Hypoxic environment was created applying a controlled hypoxic gas chamber. Prior to testing the applicability of NBQ48 as a hypoxic fluorescent marker, cytotoxic studies where performed to identify a low-toxicity dose at 24 hours under aerobic and hypoxic environments that would allow the bio-reduction process with little toxicity. The differences in fluorescence emission after treatment was measured by fluorometer and fluorescence microscopy. Results indicated that cell treatments up to 24 hours with NBQ48 under aerobic environment did not reach an IC50 dose in COLO205 cells, however under hypoxic environment the IC50 reached at 100μM. The significant fluorescence increment after 24 and 48 hrs in 2D and 3D cultures treated with NBQ48 (75uM) at hypoxic in contrast to aerobic environments clearly demonstrated the need of a low oxygen content for the bio-reduction of the parent NBQ48. This study confirms the applicability of NBQ48 as markers for hypoxia in metabolically active 2D and 3D cultures. This hydrophilic hypoxic marker could additionally aid researchers in testing hypoxia activated pro-drugs for therapeutic applications in cancer as well as on other diseases where cellular hypoxia is a significant risk factor.
Keywords: hypoxia, NBQ quinolinium salt, fluorescent marker, 3D cultures, colon cancer
BQS, benzazolo[3,2-a]quinolinium salts; IC50, 50% cell growth inhibition concentration; NBQ, 3-nitrobenzazolo[3,2-a]quinolinium salts; PBS, phosphate buffer saline; 2D,two dimensional cell culture; 3D, three dimensional cell culture
Hypoxia or low oxygen content in human tumors can limit tumor cell response to radiation and chemotherapy and predisposes tumor cells to metastasis and consequently cancer cell migration.1 Given the impact of hypoxia on cancer treatment and prognosis clinical studies to date continue to focus on strategies that can diagnose and overcome the effect of hypoxia microenvironment effects. It has been described how microenvironment within a solid tumor consists of heterogeneity on the oxygen level presenting normoxic (oxygen tension between 10-21%), hypoxic (oxygen tension between 1-5%) and necrotic areas. More specifically, for solid tumor development a relationship has been clearly established between absence of capillaries in tumor and low oxygen concentration. A more recent study indicated that despite the variation in response to treatment among cancer types, hypoxia continues to be a common factor associated with therapeutic resistance and prognosis.2–5
Most clinical trials targeting hypoxia have yielded inconclusive results to date6–11 stressing the needed to identify new clinical and research markers for tumor hypoxia.12 A marker which could accurately screen for tumor oxygenation status would provide insight on the metabolic activity of these cells as well as helping in testing hypoxia pro-drugs as therapy toward a better prognosis. Some of the challenges in hypoxia diagnostics are based on the heterogeneity of tissue oxygen levels, type of hypoxia, sample tissue volume and also type of approach (direct or indirect). Low oxygen levels can change cell behavior and is associated with changes in genetic expression and up-regulation of proteins, such as HIF-1α, facilitating migratory and metastatic behavior thereby increasing the aggressiveness and probability of an incurable solid tumor and prognosis.13
The here reported 2D and 3D cell culture in-vitro study focuses on validating the applicability of 3 nitrobenzazolo[3,2-a]quinolinium chloride salts (NBQS) as markers for metabolically active hypoxic tumor cells that could eventually be applied to better describe the tumor microenvironment and improve the prognosis of current cancer treatments.
In the search for new active molecules for treatment, it has been addressed by the scientific community11 how bioreductive drugs under hypoxic tumor microenvironment could generate reactive species or metabolites. Furthermore, it has shown that these metabolites could have the property to mark and inhibit hypoxic cells and in turn destroy the tumor if they achieve a stable microenvironment.12 It is however important to address the toxicity that some of these organic compounds could induce given their hydrophobic properties and lack of water solubility. We here present the assessment of NBQ48, member of a family of compounds reported by Cox O14 & Zayas B15 as a potential marker for hypoxic cells (Figure 1). Although the proposed NBQ compounds share some similarities to current reagents, the hydrophilicity of our NBQs could provide a more even bio-distribution on future in vivo models. In general, it is considered that lipophilic drugs can be more toxic than hydrophilic drugs especially since hydrophilic drugs can minimize in vivo crossing of blood-brain barrier or peripheral nerves effects.
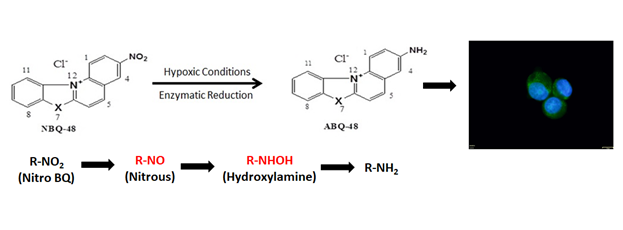
Figure 1 NBQ48 Structures and proposed bio-reduction of NBQ48 under hypoxic conditions to form a fluorescent metabolite. The fluorescent microscopy image is an actual data from colon cancer cells after treatment with NBQ-48 as describe in the result section.
Although typical 2D cell cultures are applied on in-vitro studies, a 3D tumor model can mimic even closer an in-vivo scenario toward a translational approach.16–18 In this study, COLO205 colon cancer cells were selected as model to test the ability of NBQ48 as hypoxic marker. Previously NBQ48 as well as other NBQs have been screened for their cytotoxicity using the National Cancer Institute’s (NCI) 60 human cancer cell lines, which present nine varied histologies.19 The toxicity was evaluated under aerobic conditions, but not under hypoxia. Towards this end, we have designed a set of experiments and treated colon cells under hypoxic conditions in contrast to cells treated under aerobic conditions to screen the formation of a fluorescent metabolite.
Determination of the bio reduction of NBQ48 was also assessed in this study by testing the reductive activity of Cytochrome P450 on NBQ48. The reduction was measured by the formation of a fluorescence metabolite (presumably ABQ-48) when NBQ48 in solution and the reductases were combine. The tested samples contained a combination of NBQ-48, Cytochrome P450, and ABQ-48 as the positive/fluorescent control. Cells however were not included in this experimental set.
The main purpose of the reported study, is to provide evidence of the increment in fluorescence generation, which is believed to be from a bio-reduction process under hypoxia as presented in Figure 1. The cellular green fluorescence is from the generated metabolite after incubation with NBQ48 at hypoxic conditions. Thus, our studies represent the first example of the utility of this NBQ as marker for metabolically active hypoxic cells.
Chemicals
Purified and characterized NBQ48 (7-benzyl-3-nitrobenzimidazo [3,2-a]quinolinium chloride) was synthesized14,15 in house . Stock solutions at 3mM dissolved in sterile water were prepared the day before the treatment and stored in a refrigerator at 2-5°C until used for cell treatment.
Cell cultures
Epithelial colorectal adenocarcinoma COLO205 CCL-222 tumor cells (ATCC, Manassas VA) in 2D cultures were seeded and maintained in RPMI 1640 cell culture medium supplemented with 10% fetal bovine serum (ATCC) and incubated at 37°C with humidified atmosphere of 95% air and 5% CO2. Cell viability prior and during the NBQ48 treatments was determined with an automated Countess Cell counter from Invitrogen using the presto blue (PB) exclusion method. Prior to treatment, cells were sub cultured and kept at a density of 5.0×105 cells per 3.5mL of culture media plus additives in 25cm2 flasks to assure stable metabolic state and exponential growth.
3D tumor cultures
Generation of the 3D tumor cultures was achieved by implementation of the Bio Assembler Magnetic Levitation System and as recommended by the manufacturer (n3D Biosciences). Briefly, iron nanoparticles are used for Magnetic Levitation.20 These iron nanoparticles approved by the FDA do not interfere with the compounds fluorescence emission. A minimum of 1x106 cells placed in the Bio Assembler Magnetic Levitation cell culture dish were combine with 200µL of Nanoshuttle nanoparticles (made of gold, iron oxide and poly-L-lysine) forming a homogenized culture. Cultures then are incubated at 37°C 5%, CO2, for a period of 15 minutes to an hour for the formation of 3D tumor model. Once the 3D tumors were formed, controls and NBQ48 treated, 3D cultures were then located in aerobic and hypoxic environments for 24 and 48 hours.
Enzymatic reduction of NBQ48
Compound NBQ 48 (7-benzyl-3-nitrobenzimidazo [3,2-a]quinolinium chloride) was synthesized and characterized in house.14,15 Hypoxic samples were purged of oxygen for 30 minutes using argon gas and bubbler system (except negative aerobic control). Enzymatic reduction reaction were conducted following reported references.21,22 All reagents were obtained from Sigma Aldrich (St. Louis, MO). Four distinct samples in triplicates were prepared. Briefly, the reaction consists of an 800µM NBQ 48 solution mixed with NADPH 6.4mM. Cytochrome P450 reductase at a concentration of 1.1U was included along with sodium phosphate buffer 12mM and water for a final volume 150µL and incubated for 24 hours at 37ºC. After incubation, 850µL of aerobic 50mM sodium phosphate were added and incubated one hour at room temperature, then fluorescence was measured with a GloMax fluorometer, (Promega, Sunnyvale CA ) using the blue filter. Results were tabulated using Graphpad software (Version 6). A one way ANOVA with Dunnett’s multiple comparison test was perform to prove significance, where p<0.05 is considered significant.
Hypoxic chamber validation
In order to generate a hypoxic environment for COLO205 2D and 3D cell cultures, the MIC-101 Hypoxia Incubator Chamber (BioSpherix. Ltd) was applied. Cell cultures of treated and controls, were located inside the hypoxia chamber and in order to validate the hypoxic environment, a hypoxia dry anaerobic indicator strip was included as well.23 The chamber was then sealed and connected to a nitrogen (99.9% purity) gas tank. The gas tank flow was maintained at 20 to 25 liters per minute (L/m) for a minimum of 10 to 15 minutes and then placed in the incubator for 24 or 48 hours. All experiments were performed in triplicates.
Fluorescence of 2D and 3D COLO205 cells
COLO205 cells were exposed to NBQ48 compound under hypoxic vs. aerobic environment in order to confirm the applicability of NBQ48 as a hypoxic marker. A minimum of 1x106 cells were seeded in 3mL of RPMI 1640 culture medium in 25cm2 flasks. The control group consisted of cells suspended in culture medium without NBQ48 and experimental group of cells treated with NBQ48 compound at a 75μM dose. Sets of control and treated samples were incubated at aerobic and hypoxic environments for 24 and 48 hours. The differences in fluorescence emission was then measured with the OPTIMA (BMG, Cary N.C.) fluorimeter and by fluorescent microscopy with an FSX 100 Bio Imaging System from Olympus (Center Valley P.A.). The OPTIMA fluorimeter analysis was programmed with a range of excitation wavelength emission at 355-612 nanometers (nm) for the detection of NBQ48 bio-reduced compound. In order to measure the significant difference in fluorescence between the two environments, a one way ANOVA with Dunnett’s multiple comparison test was perform to prove significance, where p<0.05 is considered significant.
Determination of IC50
COLO 205 tumor cells were seeded in 96 well plates at 50,000cells/well (100µL final volume) and treated with NBQ48 for 24 and 48 hours in experimental doses ranging from 0 to 200µM. Cell viability and IC50 determination implemented by the presto blue (PB) reagent according to the manufacturer's protocol.19 Briefly, after treatments, cells washed and incubated with 10µL of PB reagent and 90µL of medium and analyzed using fluorostar Optima (BMG) fluorescence reader. Changes in cell viability was detected with fluorescence determination at excitation 570 nm; and emission 610nm. A dose–response curve was generated and plotted using a non-linear regression analysis for the IC50 determination of treated cells.
IC50 determination
Determination of the NBQ48 IC50 concentration on cells treated at hypoxic and aerobic environment at 0 to 200µM, demonstrated that under aerobic conditions NBQ48 has no cytotoxic effects on COLO205 cells after an exposure period of 24hours. Under hypoxic conditions however, toxicity was observed with an IC50 in COLO205 cells at a concentration of 100μM. Given the cell toxicity observed under hypoxic environment, it is reasonable to consider that a bio reduced metabolite (presumably ABQ-48) possessing higher toxicity than its parent NBQ48 moiety was formed.
Fluorescence of 2D COLO205 cells
In order to determine the applicability of NBQ-48 as a novel screening tool for hypoxic tumor cells, COLO-205 were exposed for 24 hours at 75uM under aerobic versus hypoxic environments. The formation of a fluorescence metabolite was observed as early as 4hours exposure however, the difference between both environments was not statistically significant until 24 hours of exposure. These results are clearly consistent with reported evidence on multiple tumor cell lines24 where increased expression of HIF-2α factor demonstrated over a period of 24 to 72 hours under hypoxic environments (1% O2). This evidence supports the fact that a bioreduction process of NBQ48 to a fluorescent amino metabolite in COLO205 cells becomes significant after 24 hours exposure under hypoxic environment. Figure 2 clearly presents that the fluorescence emission in COLO205 2D cell cultures exposed to NBQ48 for 24 hours was three to four times higher under hypoxic environment than under aerobic environment.
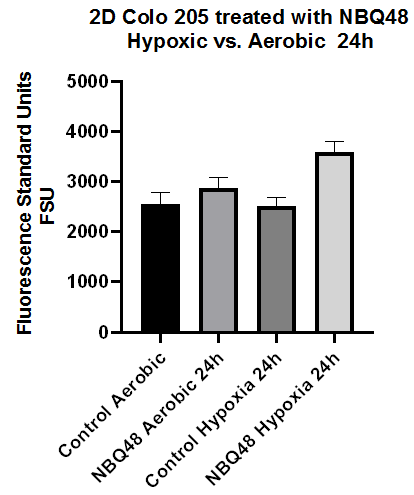
Figure 2 Fluorescence emission of 2D COLO205 cells exposed to NBQ48 (75μM) for 24 hours under hypoxia versus aerobic environment. A one way ANOVA with Dunnett’s multiple comparison test was performed to determine significance. Significant difference was observed in the fluorescence emission (adjusted p-value<0.001) of cultures treated with NBQ48 at hypoxic environment versus aerobic cultures.
After 48 hours of treatment as presented in Figure 3 this significance in fluorescence remained but at a lower intensity. Fluorescence microscopy analysis of aerobic 2D cultures also demonstrated limited fluorescence for cells exposed to NBQ48 for 24 and 48 hours in contrast to the control cells. Under hypoxia however, increased fluorescence, was clearly observe when treated with NBQ-48 after an exposure period of 24hr as observed in Figure 4 consistent with quantitative results.
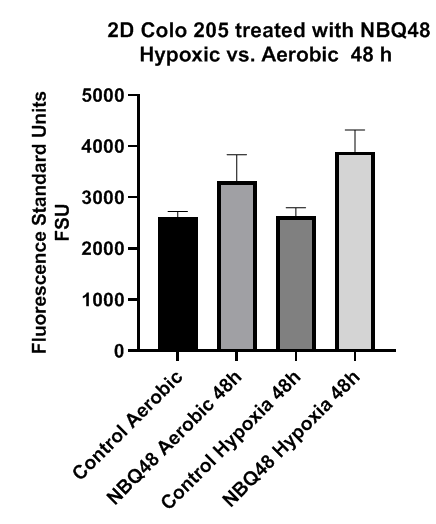
Figure 3 Fluorescence emission of 2D COLO205 cells exposed to NBQ48 (75μM) for 48 hours under hypoxia versus aerobic environment. A one way ANOVA with Dunnett’s multiple comparison test was performed to determine significance. Significant difference was observed in the fluorescence emission (adjusted p-value <0.001) of cultures treated with NBQ48 at hypoxic environment versus aerobic cultures.
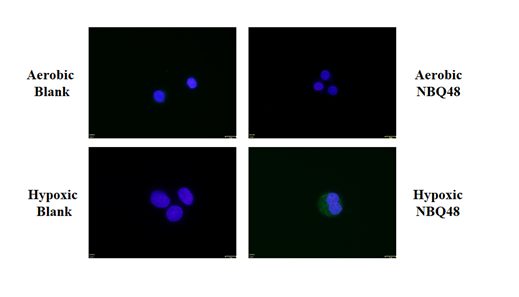
Figure 4 Fluorescent microscopy analysis of COLO 205 cancer cells treated with NBQ48 to detect fluorescent emission after 24 hours resulting from bio-reduction of NBQ48 into a fluorescent metabolite. Cell nucleus stained with Hoechst stain (blue) for imaging clarity of each cell. A) Aerobic cells w/o NBQ48; B) Aerobic cells with NBQ48; C) Hypoxic cells w/o NBQ48; D) Hypoxic cells with NBQ48. The greenish/fluorescent cells on D, indicates the hypoxic formed metabolite.
Furthermore, to establish the reduction activity on hypoxic tumor cells and the formation of a fluorescent metabolite, we screened enzymes that may be responsible for the reduction and formation of the fluorescent species observed in the experimental cultures. Results as shown in Figure 5 that samples with NBQ48 but without the reductase, generated limited (background) fluorescence. Limited fluorescence was also seen in, samples with NBQ48 and enzymes but under aerobic conditions (aerobic samples). Only experimental samples under hypoxia, NBQ48 and Cytochrome P450 reductase had fluorescent levels close to the positive control (ABQ standard) clearly establishing that these reductases are acting upon NBQ and reducing it into the fluorescent metabolite. All values were background corrected to account for baseline fluorescence. The experimental sample containing NBQ48 presented average values of 1486.68 FSU (Fluorescence Standard Units). This value presents a statistically significant (P<0.05) difference when compared to the negative control (reaction vehicle, no enzyme) which presented an average value of 111.40 FSU. An additional negative control sample consisting of the complete reaction in an aerobic environment presented values comparable to the vehicle blank thus indicating that the reaction did not occur in the presence of oxygen. The positive sample consisted of reaction vehicle spiked with the presumable product in the experimental samples (ABQ48 the reduced form of NBQ48) this sample presented the highest average fluorescence values with 2476.73 FSU.

Figure 5 Comparison of fluorescence emission after enzymatic reduction of NBQ48 by Cytochrome P450. Average fluorescence values (FSU) per sample as follows: Blank 111.40FSU, Aerobic negative sample 158.96FSU, positive ABQ spike 2476.73FSU and NBQ48 experimental of 1486.68FSU.
Is important to put in perspective that the fluorescence observed in 2D COLO205 cells treated with NBQ48 under a hypoxic environment is consistent with the above presented experiment with NBQ48 in the presence of reductases showing how oxido-reductases, such as CYP450 enzymes are mediators in drug activation under hypoxic environment.25 Furthermore, it have been shown that in colon cancer there is a high expression of the oxido-reductase enzyme CYPZW1 in advanced phase solid tumors.
3D tumor model
For a more tumor resembling model 3D cell culture were also prepared and treated with NBQ48. These 3D cultures showed consistent tissue tumor models” formation after 24hours of assembly. As in 2D cultures stronger fluorescence emission was observed after 24hours of exposure to NBQ48 (75uM) under hypoxic environment compared to aerobic environment as presented in Figure 6.
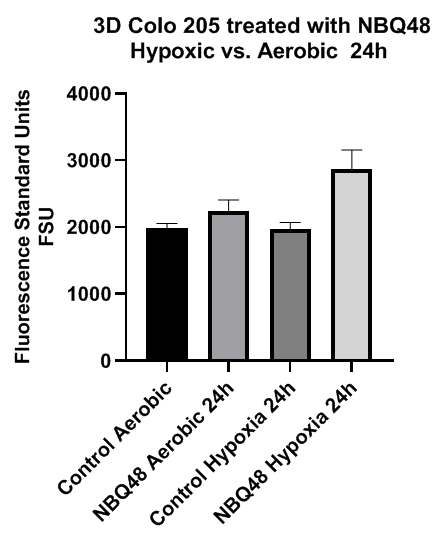
Figure 6 Fluorescence emission of 3D COLO205 cells exposed to NBQ48 (75μM) for 24 hours under hypoxia versus aerobic environment. A one way ANOVA with Dunnett’s multiple comparison test was performed to determine significance. Significant difference was observed in the fluorescence emission (adjusted p-value <0.001) of cultures treated with NBQ48 at hypoxic environment versus aerobic cultures.
In contrast to the 2D cultures, significant difference was not observe on fluorescence emission at 48 hours treatment of the 3D cultures between hypoxic and aerobic environments. Fluorescent microscopy images were also consistent with significant fluorescence emission after 24 hours of hypoxic NBQ48 treated 3D cultures in contrast to aerobically treated 3D cultures as demonstrated in Figure 7. Cells were also counter stained with Hoechst 33342 for cell nucleus visibility.
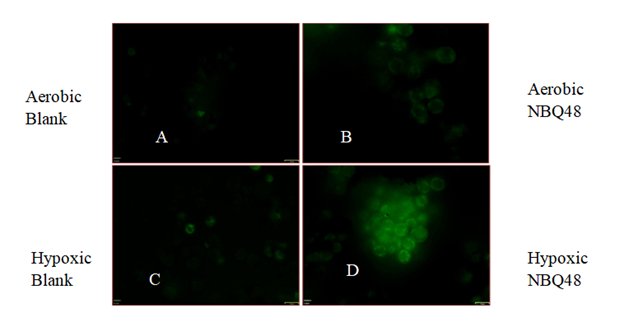
Figure 7 Fluorescent microscopy analysis of 3D COLO 205 cancer cells treated with NBQ48 to detect fluorescent emission after 24 hours resulting from bio-reduction of NBQ48 into a fluorescent metabolite. A) Aerobic cells w/o NBQ48; B) Aerobic cells with NBQ48; C) Hypoxic cells w/o NBQ48; D) Hypoxic cell with NBQ48. The greenish/fluorescent cells on D, indicates the hypoxic formed metabolite. Minimum auto fluorescence (background) of colon cells is also observed in images of aerobic samples A, B.
The need for probes or compounds that can selectively and efficiently detect hypoxia in vivo is of high importance and requires attention. This study reports the assessment of NBQ48 as a marker for hypoxic tumor cells in 2D and 3D cell cultures. The hypoxic environment implemented to maintain the low level of oxygen in order to create a more cell type relevant hypoxic environment simulated oxygen-deprived cells found on solid tumors. More specifically, NBNQ48 as a hypoxic marker was tested with colon cancer cells (COLO 205) where solid tumors has been detected. The presented study demonstrated the generation of significant fluorescence from bio reduced formed metabolite(s), on treated cultures at 24 and 48hrs under hypoxic environments clearly confirming our original hypothesis.
The enzymatic reduction activity experiments demonstrated how the cytochrome P450 reductase catalyzes the reduction of the nitro containing NBQ compound to its amino substituted analog ABQ48. Previous work26,27 demonstrated the potential of NBQ compounds to be reduced by the hypoxanthine and xanthine oxidase enzymes and includes the formation of a hydroxylamine intermediate. This reduction promotes the formation of BQ and DNA base adduct (BQ and 2′-dG) which have been detected previously. Interestingly, reduction by cytochrome P450 reductase likely promotes the formation of a hydroxylamine intermediate characteristic of the reduction of the nitro substituted BQS compounds. This work provides evidence of the reduction potential of another key metabolic enzyme, cytochrome P450 reductase a key enzyme in the metabolism of pro-drugs and xenobiotic substances.
2D and 3D cultures treated with NBQ48 demonstrated fluorescence emission depended on the reduction of the NBQ48 in cells treated with 75uM for 24 hours under aerobic and hypoxic environments. Fluorescence values are clearly higher for cells treated under hypoxic environment indicative of the formation of the reduced (fluorescent) metabolite. A one way ANOVA with Dunnett’s multiple comparison test was performed to determine significance.
Qualitative data determined with fluorescent microscopy (Olympus FSX100 Fluorescence microscope) of COLO 205 treated for 24 hours with NBQ48 at hypoxic and aerobic conditions clearly showed increment in the green fluorescence cellular regions at hypoxic conditions in contrast to cells at aerobic conditions.
This study showed the applicability of NBQ48 in 2D and 3D COLO205 cells to identify tumor cells under hypoxic environment. The changes in fluorescent emission levels as well as fluorescence microscopy images demonstrated the need of a reduced oxygen environment for the formation of a fluorescent metabolite. For further confirmation of the applicability of other NBQs on multiple cell types, an expanded study on varied cancer cells as well as correlations with the expression of cellular hypoxia indicators such as HIF-1α factors is being developed.
This project was supported in part by grants from the National Institute of General Medical Sciences (5 P20 GM103475-16) from the National Institutes of Health.
Authors declare no conflict of interest.

©2020 Zayas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.