Journal of
eISSN: 2373-633X


Research Article Volume 8 Issue 3
1Head of the nuclear medicine service, National Institute of Respiratory Diseases, Mexico
2Third year Resident in Nuclear Medicine, National Institute of Respiratory Diseases Mexico
3First year Resident in Nuclear Medicine, National Institute of Respiratory Diseases Mexico
Correspondence: , Tel 91-11-26546665
Received: July 26, 2017 | Published: August 17, 2017
Citation: de León JMSP, Flores PJ, Zepeda PIF. Neuroendocrine neoplasms: a case report with complete reverse mismatch and the correlation with differentiation rate. J Cancer Prev Curr Res. 2017;8(3):279-281. DOI: 10.15406/jcpcr.2017.08.00280
Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors with an unpredictable expression that makes diagnosis and treatment difficult.1 A 68-year-old man with history of 3-month weight loss and rapid gastric fullness comes to the clinic. The physician indicates abdominal tomography, ultrasound and endoscopy with biopsy of the upper digestive tract where an infiltrating lesion is discovered in the greater curvature of the stomach, immunohistochemistry reports carcinoid tumor. The patient is then sent to the Nuclear Medicine service seeking Peptide Receptor Radionuclide Therapy (PRRT). A PET/CT scan is performed using 68Ga-DOTANOC, with another one a day later with 18F-FDG to assess whether he is a candidate for said treatment. The images show a complete reverse mismatch pattern in the liver metastasis and primary gastric tumor found, making PRRT not an option due to a high degree of dedifferentiation and low expression of somatostatin receptors in such lesions.
Keywords:neuroendocrine neoplasms, 18F-FDG, 68Ga-DOTANOC, complete reverse mismatch
Neuroendocrine neoplasms are a histological strain that tends to have a very unusual behavior compared to other types of tumors; they are derived from neuroendocrine cells which are very abundant in the digestive tract.2 Its slow growth coupled with non-specific and non-alarming symptomatology gives it the ability to grow and metastasize before the diagnosis is made.3 The degree of differentiation demonstrates the expression of somatostatin receptors, both being directly proportional.4 Immunohistochemistry with Ki-67 and mitotic index are the two methods that assess the differentiation degree of the tumor from which the biopsy was taken (Figure 1),4 however the biological heterogeneity of this type of lesions and their metastases make it difficult to assess the tumor burden. There are markers such as chromogranin A, synaptophysin and specific neuronal enolase but although highly sensitive they have a low specificity since they are often altered by other conditions other than neuroendocrine neoplasms. PET/CT molecular imaging studies performed with 68Ga somatostatine analogs have proven useful for estadification, re-estadification and assessment of treatment response and prediction of outcome due to their high spatial resolution, wide spread scan and high specificity thanks to their coupling with the receptor expressed on the membrane of these tumors. It is necessary to keep in mind neuroendocrine neoplasms’ highly unpredictable expression of said receptors, so it is advisable to complement this study with a 18F-FDG PET/CT scan since the dedifferentiated cells have a greater glycolytic metabolism, allowing a glimpse into prognosis.
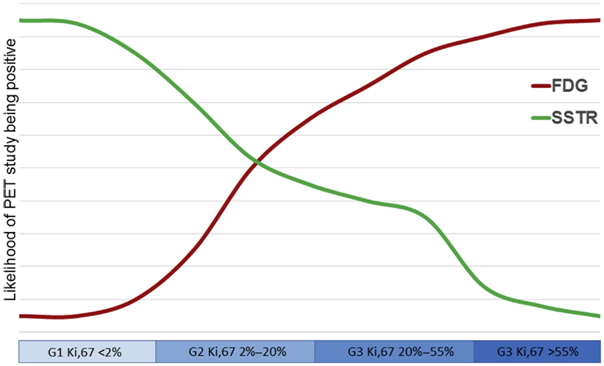
Figure 1 Likelihood of positive SSTR and FDG PET/CT depending on proliferative rate in patients with neuroendocrine tumors. Most patients with indolent well-differentiated tumors have an SSTR-positive FDG-negative disease (mismatch), whereas patients with aggressive, poorly differentiated carcinomas tend to SSTR-negative FDG-positive disease (mismatch pattern, existing complete and partial forms). Patients in the middle of the spectrum have both SSTR- and FDG positivity (match pattern, also with complete and partial forms). Reproduced with permission from the authors: Pattison D, Hofman M.
A 68-year-old male began a year ago with early gastric fullness, hyporexia, astenia, constipation and a 3-month long weight loss, shedding 10 kg in the last month, accompanied by intermittent abdominal pain that is not disabling. His physician requested abdominal ultrasound, contrasted abdominal CT and endoscopy with biopsy of the upper digestive tract, finding an infiltrating, friable, ulcerated lesion in the major curvature of the stomach extending to the proximal antrum (Figure 2). Immunohistochemistry was positive for chromogranin A and cytokeratin thus diagnosing NEN (Figure 3). The contrasted abdominal computed tomography reports solid images in the liver, probably in relation to hemangiomas but not ruling a metastatic process out either. The patient does not accept conventional chemotherapy so he goes to the National Institute of Respiratory Diseases to receive Peptide Receptor Radionuclide Therapy (PRRT). Initially, a review of the lamellae was requested with quantification of Ki-67 percentage which showed a proliferation index of 70%, and a chromogranine A serum test of 1266 ng/mL (normal <93 ng/mL). The next day the patient got a 68Ga-DOTANOC PET/CT scan and a second one using 18F-FDG the day after. The primary stomach tumor and several hepatic lesions were found, the largest of which were located in segments VI, VII and VIII. None showed any avidity for the radiolabeled somatostatin analog, but the 18F-FDG PET/CT revealed that these same lesions had a high metabolic rate as reflected by the high concentration of radiotracer. This pattern is known as complete reverse mismatch.
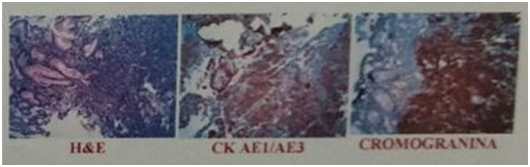
Figure 3 Immunochemistry stains that show cytokeratin (++) and chromogranine (+++), findings consistent with a high grade NEN.
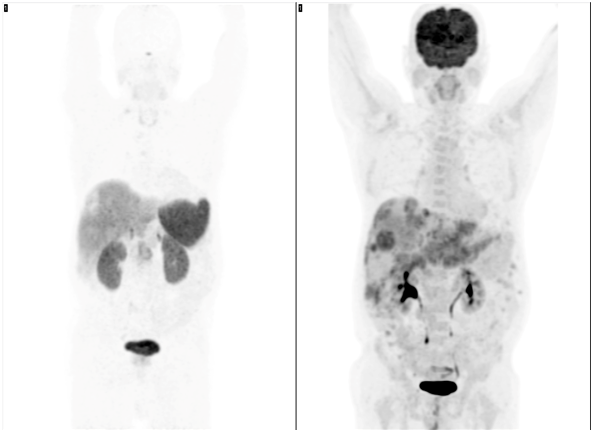
Figure 4 PET/C Tanterior Maximum-intensity projection (MIP). Left: 68Ga-DOTANOC heterogeneous uptake in the liver with some photopenic areas (arrows). Right: 18F-FDG shows multiple increased uptake foci in the liver metastases and a large uptake inferior to the left hepatic lobule consistent with primary gastric tumor.
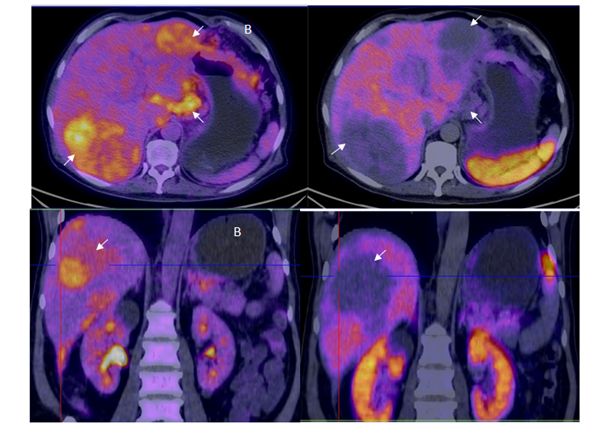
Figure 5 A: Axial and coronal 18F-FDG PET/CT fusion showing two large hipermetabolic foci in both hepatic lobes as well as the mass visible in the lesser curvature of the stomach consistent with the primary tumor (arrows). B: Same projections in axial and coronal PET/CT using 68Ga-DOTANOC, without uptake using the somatostatine analog in the hepatic metastases and gastric tumor, resulting in the pattern known as reverse mismatch which is indicative of lesion dedifferentiation and consequent poor prognosis.
NENs’ expression of somatostatin receptors confers the ability to bind somatostatin, a feature useful for prognosis, prediction, follow-up and treatment assessment. PRRT takes advantage of the expression of these receptors to target its highly energetic particles and thus have a localized therapeutic effect. However, not all patients are candidates for this type of therapy since only well and moderately differentiated NENs will have a favorable response. PET/CT studies with 68Ga tagged somatostatin analogs and 18F-FDG have the advantage of assessing the degree of differentiation not only of the primary lesion but its metastasis as well, which is comparable with Ki-67 immunohistochemistry in vivo, simultaneously evaluating the differentiation of all lesions observed by these functional and anatomical imaging studies.
Currently, NENs have gone from an uncommon entity to a more frequent diagnosis. According to the WHO, by 2010 the age-adjusted incidence of small intestine and digestive system carcinoids had increased by 460% and 720% respectively over the past 30years,5 these numbers do not suggest an increase in incidence but a decrease in underdiagnosis instead. Previous concepts of this disease catalogued it as only rarely potentially malignant and with favorable prognosis, however recent studies have refuted this idea. Not only do NENs have a slow and indolent spread and de-differentiate both the primary lesion and its metastasis, but the difficulty of detecting viable tumor cells using conventional diagnostic studies makes it easy for the lesion to go unnoticed. Fortunately Diagnostic and Therapeutic Nuclear Medicine has begun solving these problems, not only with diagnostic and follow-up PET-CT scan with 68Ga attached to somatostatin analogues, but with PRRT serving as a vehicle to transport therapeutical beta-emitting radionuclides such as 90Y and 177Lu. It holds the benefit of killing cells in neuroendocrine tumors while being a therapeutic strategy directed only to cells expressing somatostatin receptors, sparing healthy ones. The present and future trend in oncological care heads towards personalizing treatments in order to reduce the side effects of current available therapies and individualizing management to each patient. And this is where the Nuclear Medicine Therapeutics is a cornerstone in achieving the objective.6–17
The authors declare that there is no conflict of interests regarding the publication of this paper.
None.
None.

©2017 de, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.