Journal of
eISSN: 2373-633X


Review Article Volume 5 Issue 6
International Institute of Medicine and Science, California, USA
Correspondence: Alain L Fymat, International Institute of Medicine and Science, California, USA, Tel (760) 485-9149
Received: August 25, 2016 | Published: September 20, 2016
Citation: Fymat AL. Nanotechnology and cancer.J Cancer Prev Curr Res. 2016;5(6):347‒353. DOI: 10.15406/jcpcr.2016.05.00180
To help meet the goal of palliating suffering and eliminating death due to cancer, the U.S. National Cancer Institute (NCI) has, and continues to be, engaged in a concerted effort to harness the power of nanotechnology to radically change the way we prevent, diagnose and treat cancer. Using both in-house and outside inputs from professional stakeholders (clinicians, cancer researchers and technologists, biologists, geneticists, and others), the NCI has developed its Cancer Nanotechnology Plan (so-called “CaNanoPlan”) to meet the above goal. The Plan lays out a pathway and a set of mechanisms to facilitate nanotechnology becoming a fundamental driver of advances in cancer research.
During the due diligence phase in the development of the CaNanoPlan, NCI noted the significant obstacles that needed to be overcome to transition nanotechnology to clinical application. These obstacles centered around three common themes:
To address and overcome these barriers, the NCI established the Nanotechnology Characterization Laboratory (NCL) at its NCI-Frederick facility. The NCL is a formal collaboration with the U.S. National Institute of Standards and Technology (NIST) and the U.S. Food and Drug Administration (FDA). Its charter is “to perform the pre-clinical characterization of nanomaterials and to facilitate the development of nanomaterials intended for clinical use”. Focused development and pre-clinical testing of new technologies by the NCL will facilitate entry of these products into clinical trials, and this will directly affect the number of new diagnostic and treatment strategies that reach the market, i.e., new drug approvals.
The NCI's CaNano Plan, published in 2004, derived from the NCI Alliance for Nanotechnology in Cancer (ANC). It was a 10-year plan launched on the premises that:
The new CaNanoPlan, published in 2010, summarizes the present state of significant areas in the field and builds upon recent discoveries. It is an ensemble of individual reports and strategic plans by several investigators who participated in Phase I of the program. It also incorporates the opinions voiced at the series of strategic meetings held at NCI. Each chapter presents the current status of development; highlights avenues for growth and opportunity; elucidates clinical applications for the technologies; and forecasts what goals might be achieved in the next 3-10years. For fuller details, the interested reader is referred to Grodzinski et al.,1 and the references therein.
The areas covered included in particular:
Here, I will address only a few of the above areas while focusing on the use of nanotechnology to combat cancer.
It must first be noted that biological processes, including ones necessary for life and those that lead to cancer, occur at the nanoscale. Thus, in fact, we are composed of a multitude of biological nano-machines (Note: Comparative sizes (in nanometers nm) of several chemical and biological molecules are: glucose molecule ~1, antibodies ~10, viruses ~102, bacteria ~103, cancer cells ~104-105). The domains of application of nanodevices: nanopores, dendrimers, nanotubes, quantum dots, nanoshells, etc, are ~10-102). Accordingly, nanotechnology provides researchers with the:
Nanotechnology also offers important benefits for diagnosis by providing:
Still further, regarding treatment and clinical outcomes, considering that cancer therapies are currently limited to surgery, radiation and chemotherapy, which risk damage to normal tissues or incomplete eradication of the cancer, nanotechnology offers the means to:
Cancer cells are notoriously good at becoming resistant to the drugs meant to kill them. One way they do this is by rerouting their signaling networks, specifically those responsible for the cancer cells' growth, proliferation, and survival. For example, doctors may give a drug to block a particular signaling pathway, but within a matter of days-and in some cases minutes-cancer cells begin to rely on an alternate pathway to promote their survival. To combat this, patients are often given multiple drugs, so-called “rational combination therapy”, in the hopes of attacking both primary and alternative pathways, the goal being to preemptively block the cancer cell’s escape route. Yet, control of the above process when more than one drug will enter a cell and how much of each will enter has been a significant challenge that has limited the efficacy of many combination therapies. The reason is that drugs often have very different chemical properties, which cause them to travel to different parts of the body and enter cancer cells at different rates. For example, drugs that are hydrophilic, or water-loving, tend to accumulate in tissues with high water content, whereas hydrophobic drugs, which are repelled by water, steer clear of these same areas.
Targeting of cancer cells by nanoparticles
The layer-by-layer nanoparticle consists of three components:
The purpose of the multilayered shell is to stabilize the particles, prevent drug leakage, target the particles to the slightly acidic environment of the tumor, and minimize the particle’s interactions with non-cancerous cells. The multilayered shell can also be used to transport drugs that are not easily stored in the core, such as highly charged nucleic acids. These molecules can be spread out within different layers of the shell so that they are separated from drugs that can inactivate their therapeutic effects, for example, platinum drugs. The exterior layer contains molecules that further target the nanoparticles to cancer cells and help them pass through the body unnoticed by the immune system. The above approach may be applicable to several types of cancer since the targeted pathways are present in a variety of tumors, which makes the result particularly significant. Figure 1 shows nanoparticles (yellow) targeting and entering cancer cells (blue).
The SERS nanotag
A team of researchers at the University of Cincinnati discovered a new nanostructure called a “surface-enhanced Raman spectroscopy (SERS) nanotag” that showed significantly better properties for use in cancer detection and treatment. The technique targets molecules using lasers, which results in a scattering of light at different wavelengths along a spectrum. Because the molecules produce weak signals, gold or silver nanoparticles are usually employed to amplify the signals followed by measurement by a Raman spectrometer for analysis. The process is highly sensitive but fraught with challenges, including difficulties with reproducibility, signal stability and apparently a lack of quantitative information although Raman spectral quantities (line frequency center, line spectral width, four-dimensional spectral polarization vector) could theoretically provide the needed information.
The researchers combined two previously known concepts:
They thus created a structure comprised of a smooth inner metallic core surrounded by a spiky metallic outer shell with three nanometer spacing. This newly created nanotag produced a X10 times greater signal enhancement compared to smooth-shell core structures, making it possible to detect minute amounts of organic molecules, such as DNA for particular diseases. The spiky structures are also more efficient at generating heat that can be used to destroy cancer cells. They additionally offer an increased surface area that can accommodate more drugs in order to deliver a greater targeted blast to diseased cells allowing targeting, image and release drugs all with one device.3
In situ nanoparticle vaccination
Nanotechnology has a tremendous potential to contribute to cancer immunotherapy. Scientists at Case Western University and Dartmouth University have recently (December 2015) revived and tested a 100-year-old idea called “in situ vaccination” in mice with the intent of extending this research into animal models of humans. This immunotherapy strategy directly manipulates identified tumors to overcome local tumor-mediated immunosuppression and subsequently stimulates systemic anti-tumor immunity to treat metastases. The “something” is placed inside a tumor to disrupt the environment that suppresses the immune system, thus allowing the natural defense system to attack the malignancy. That something, the hard coating of cowpea mosaic virus (CPMV, a common plant virus), inhaled into a lung tumor or injected into ovarian, colon or breast tumors, not only triggered the immune system to wipe out the tumors, but provided systemic protection against metastases. Further, it did not cause any detectable side effects as is common with traditional therapies and some immunotherapies, the reason being that everything is done locally. The CPMV nanoparticles are stable, nontoxic, modifiable with drugs and antigens, and their nanomanufacture is highly scalable. These properties, combined with their inherent immunogenicity and demonstrated efficacy against a poorly immunogenic tumor, make them an attractive and novel immunotherapy against metastatic cancer. The CPMVs are not cytotoxic as there is no RNA involved or lipopolysaccharides that may be used as adjuvants. They are not irritants whereas harsh side effects (fatigue, pain, flu-like symptoms, etc.) are more common with chemotherapy and radiation therapy and with some immuno-stimulation drugs.
The particles are shockingly potent, easy to make and do not need to carry antigens, drugs or other immuno-stimulatory agents on their surface or inside. It is not yet clear how the virus shells stimulate the immune system. Unlike most other adjuvant, the virus shells stimulate neutrophils, a type of white blood cell whose role is not yet known. In any event, they proved effective against ovarian, breast and colon tumor models. Most of the tumors deteriorated from the center and collapsed. The systemic response prevented or attacked metastatic disease.
According to the NCI, the ability of the immune system to detect and destroy abnormal cells is thought to prevent many cancers; but when tumors start to develop, they can shut down the system, allowing tumors to grow and spread. To restart immune defenses, the tumor itself is used as if it were the antigen in a vaccine, that is, the target for antibodies produced by the immune system. With its infectious components removed, the CPMV shell acts as the adjuvant, a substance that triggers and may enhance or prolong antigen-specific immune responses.
Nanoscale magnets offer a new way to find faint early traces of cancer in patients. They make use of magnetic relaxometry signals from iron-oxide nanoparticles that find and attach themselves to cancerous cells. Such cancer signals would never be detected by X-rays. The research was carried out at Rice University by a research team led by Rivière B4 doctors at the University of Texas MD Anderson Cancer Center in Houston, TX and other senior scientists based in Albuquerque, NM. Today’s best cancer detection methods only catch tumors with more than 10million cancer cells. This new approach has the potential to detect tumors with as few as 20,000 cells.
All magnets (or materials prone to magnetism) have magnetic “moments” like invisible needles that can move and react to magnetic fields, even if their physical hosts cannot. When exposed to an external magnetic field, these ghostly needles align with it; when the field is removed, they “relax” once again. Relaxometry measures this latter characteristic. This is the very principle of magnetic resonance imaging (MRI). It turns out the moments relax at a very different rate when they belong to nanoparticles that are bound to cancer cells.
The 25-nanometer superpara magnetic iron-oxide nanoparticles are enhanced with antibody proteins that target biomarker proteins produced by cancer cells. Once they bind to the cells, their range of motion is severely restricted, and this restricted movement is pretty important. Upon applying an external magnetic field, the particles’ dipoles will align to counteract the field. Once the dipoles face each other, the net magnetic field is essentially zero. Quantifying this relaxation phase marks the location of cancer cells. This approach was tested in the laboratory and in mice. Unbound nanoparticles will randomly reorient themselves in less than a millisecond, but because antibody-associated nanoparticle complexes that are bound to cancer cells are restricted in their movement, their magnetic relaxation is a lot slower (up to a second).
The development of miniaturized systems loaded with life-saving drugs may revolutionize the way in which cancer is treated with chemotherapy, reducing the debilitating side effects of the therapy, making medications more effective, and all the while preserving the healthy living cells. (The same systems could likewise be used for delivering clot-busting drugs to the brain.)
Protein cages
Washington State University researchers have recently (October 2015) developed a unique, tiny protein cage to deliver chemotherapeutic chemicals directly to cancer cells. Direct delivery could improve treatment and lessen what can be horrendous side effects from toxic drugs. The drug delivery system uses apoferritin, the same ball of natural proteins that carries iron around in blood without letting the iron leak out. Apoferritin is made of 24 pieces that can conveniently open and close, depending on the surrounding acidity. While some research has been done on using apoferritin for drug delivery, this is the first time it was used to target lung cancer cells.
For lung cancer, the cage's exterior was modified with a ligand (a signal triggering molecule) to render the cage particularly attractive to a common cancer cell receptor. The anticancer drug daunomycin was subsequently inserted into the cage. With the addition of a small amount of acid and adjusting the pH to below neutral, the protein cage slightly opened and let the drug jump inside, where it stayed until it came in contact with the cancer cell. When the ball of drugs entered the acidic environment of the cancer cell, the cage opened and delivered the drug directly. It was determined that the ligand-guided protein cages selectively penetrated and killed more than 70 percent of the cancer cells while, contrary to typical methods of drug delivery used in chemotherapy, the system did not attack healthy lung cells.
The above system was shown to work nearly as well as - or in some cases better than-when the drug was freely moving, the type of scenario that causes the commonly experienced cancer treatment side effects. Nonetheless, these results remain preliminary and more research and animal experimentation are needed prior to using the protein cage in the clinic.
Micro bubbles
Researchers at Oxford University followed an analogous approach using “micro bubbles” the size of a 1/100th of a human hair. The micro bubbles are tiny balls of gas enclosed in an ultrathin layer of fat. They developed a way to put drugs into microscopic bubbles which can be injected into the blood stream. Theoretically, when the bubbles reach the unhealthy part of the body, they are burst with ultrasound waves, releasing the drug exactly where it is needed. Because the entire blood stream is not being flooded with the drug, side-effects from chemotherapy including hair loss and nausea can be greatly reduced. However, this concept has not yet been tested and may not be rolled out before two or more years (2018 or beyond). Two clinical trials are under way to prove the concept by testing the safety of the micro bubbles themselves and testing the effect of ultrasound on a particular chemotherapy drug. (Another use for micro bubbles would be for stroke patients by delivering clot-busting drugs directly to the right area.)
Multi-Shell hollow nanogels with responsive shell permeability
Several "nano-carriages" for drug delivery to the right address have been created but many challenges remain, chief among them being how not to let the medicine act before it gets to the right place in the body. The carriers usually encapsulate drugs through long-range electrostatic interactions wherein the carrier attracts oppositely charged medicine. Other tools are available to trigger the release of drugs, for example, an external magnetic field, different pH values, etc. But, in each case, researchers face the problem of efficiency of the drug release.
Departing from this schema and irrespective of any electrostatic force (i.e., whether the medicines are either charged or neutral), Professor Igor Potemkin and his team at the Lomonosov Moscow State University rather advocate filling gel nano-capsules by the guest molecules, locking the latter in the cavity and releasing them under temperature control. The use of gel nano-capsules is not new but it was discarded because they stick together with their neighbors (lost colloidal stability) when trying to "upload" drugs. Such behavior made the delivery impossible (or ineffective).
Potemkin and his colleagues solved this problem by creating a carrier surrounded by two "membranes" (or shells) of different chemical structures around a silica core which, at the end of the synthesis, will be chemically dissolved leaving only the “empty space” (cavity). The outer porous shell plays a protective (stabilizing) role and hinders aggregation of the nano-capsules, while the pores of the inner shell can open and close depending on the temperature due to the variable interactions between its monomeric units. At the time of filling, the pores of both shells are open and the nanogel absorbs the drug molecules as a sponge. Then, the temperature changes and the pores of the inner shell close locking in the cavity and readying the drug for delivery. Subsequently, the pores will open again and the guest molecules will be released only in the places where the temperature allows. However, here too, much work remains to be done to demonstrate that gel nano-capsules are the ideal drug delivery carrier (Figure 2).

Figure 2 Multi-Shell Hollow nanogels with responsive shell permeability. (From Prof. Igor Potemkin, Moscow State University).
Nanocarriers may carry new hope for brain cancer therapy
Glioblastoma multiform (GBM), a cancer of the brain also known as "octopus tumors" because of the manner in which the cancer cells extend their tendrils into the surrounding tissue, is virtually inoperable, resistant to therapies, and always fatal, usually within 15months of onset. Each year, GBM kills approximately 15,000 people in the United States. One of the major obstacles to treatment is the blood brain barrier (BBB), the network of blood vessels that allows essential nutrients to enter the brain but blocks the passage of other substances. What is desperately needed is a means of effectively transporting therapeutic drugs through this barrier.
Dr. Ting Hu, a nanoscience expert at Lawrence Berkeley National Laboratory (Berkeley Lab) may have the solution in the form of a new family of nanocarriers formed from the self-assembly of amphiphilic peptides and polymers, called "3HM" for coiled-coil 3-helix micelles. At only 20 nanometers in size and featuring a unique hierarchical structure, these new 3HM nanocarriers meet all the size and stability requirements for effectively delivering therapeutic drugs to GBM tumors. Amphiphiles are chemical compounds that feature both hydrophilic (water-loving) and lipophilic (fat-loving) properties. Micelles are spherical aggregates of amphiphiles. Using the radioactive form of copper (Cu-64) in combination with positron emission tomography (PET) and magnetic resonance imaging (MRI), Dr. Hu and his team demonstrated that 3HM can cross the BBB and accumulate inside GBM tumors at nearly double the concentration rate of current FDA-approved nanocarriers (Figure 3).
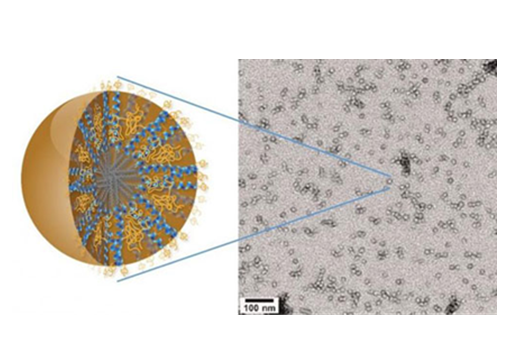
Figure 3 New 3HM nanocarriers for effectively delivery of therapeutic drugs to brain cancer tumors. (From Dr.Ting Hu, Lawrence Berkeley National Laboratory).
The 3HM nanocarriers have shown very good attributes for the treatment of brain cancers in terms of long circulation, deep tumor penetration and low accumulation in off-target organs such as the liver and spleen. The fact that they are able to cross the BBB of GBM-bearing rats and selectively accumulate within tumor tissue, opens the possibility of treating GBM via intravenous drug administration rather than invasive measures. While there is still a lot to learn about why 3HM is able to do what it does, so far all the results have been very positive.
Glial cells provide physical and chemical support for neurons. Approximately 90-percent of all the cells in the brain are glial cells which, unlike neurons, undergo a cycle of birth, differentiation, and mitosis. Undergoing this cycle makes glial cells vulnerable to becoming cancerous. When they do, they can take on different shapes, which often make detection difficult until the tumors are dangerously large. The multiple shapes of a cancerous glial cell also make it difficult to identify and locate all of the cell's tendrils. Removal or destruction of the main tumor mass while leaving these tendrils intact is ineffective therapy.
Although there are FDA-approved therapeutic drugs for the treatment of GBM, these treatments have had little impact on patient survival rate because the BBB has limited the accumulation of therapeutics within the brain. Typically, GBM therapeutics is ferried across the blood brain barrier in special liposomes that are approximately 110 nanometers in size. By contrast, the 3HM nanocarriers are only about 20 nanometers in size. Their smaller size and unique hierarchical structure afforded the 3HM nanocarriers much greater access to rat GBM tumors than 110-nanometer liposomes. Copper-64 is used to label both 3HM and liposome nanocarriers for systematic PET and MRI studies to find out how a nanocarrier's size might affect the pharmacokinetics and biodistribution in rats with GBM tumors. The results not only confirmed the effectiveness of 3HM as GBM delivery vessels, they also suggest that PET and MRI imaging of nanoparticle distribution and tumor kinetics can be used to improve the future design of nanoparticles for GBM treatment.
Conventional chemotherapy employs drugs that are known to kill cancer cells effectively. But these cytotoxic drugs kill healthy cells in addition to tumor cells, leading to adverse side effects such as nausea, neuropathy, hair-loss, fatigue, and compromised immune function. Nanoparticles can be used as drug carriers for chemotherapeutics to deliver medication directly to the tumor while sparing healthy tissue. Nanocarriers have several advantages over conventional chemotherapy. They can:
Nanoparticle drugs can be targeted either passively or actively or a combination thereof.
Passive targeting
There are now several nanocarrier-based drugs on the market, which rely on passive targeting through a process known as "enhanced permeability and retention". Because of their size and surface properties, certain nanoparticles can escape through blood vessel walls into tissues. In addition, tumors tend to have leaky blood vessels and defective lymphatic drainage, causing nanoparticles to accumulate in them, thereby concentrating the attached cytotoxic drug where it is needed, protecting healthy tissue and greatly reducing adverse side effects.
Active targeting
On the horizon are nanoparticles that will actively target drugs to cancerous cells, based on the molecules that they express on their cell surface. Molecules that bind particular cellular receptors can be attached to a nanoparticle to actively target cells expressing the receptor. Active targeting can even be used to bring drugs into the cancerous cell, by inducing the cell to absorb the nanocarrier. Active targeting can be combined with passive targeting to further reduce the interaction of carried drugs with healthy tissue. Nanotechnology-enabled active and passive targeting can also increase the efficacy of chemotherapy, achieving greater tumor reduction with lower doses of the drug.
Destruction from within cells
Moving away from conventional chemotherapeutic agents that activate normal molecular mechanisms to induce cell death, researchers are exploring ways to physically destroy cancerous cells from within. One such technology - nanoshells is being used in the laboratory to thermally destroy tumors from the inside. Nanoshells can be designed to absorb light of different frequencies, generating heat (hyperthermia). Once the cancer cells take up the nanoshells (via active targeting), scientists apply near-infrared light that is absorbed by the nanoshells, creating an intense heat inside the tumor that selectively kills tumor cells without disturbing neighboring healthy cells. Similarly, new targeted magnetic nanoparticles are in development that will both be visible through magnetic resonance imaging (MRI) and can also destroy cells by hyperthermia (see above paragraphs for further details).
Chemotherapy -the use of anti-cancer drugs (chemotherapeutic agents) to fight cancer - is often the only medical course of action in fighting cancer. While it is often the only way, it is not the best way. The problem with chemotherapy is that the very strong attack chemicals used have no way of differentiating between healthy and sick cells, so they attack all targeted cells. However, in 2015, BioSight, an Israeli medical technology start-up, has developed a technology that enables the delivery of modified chemotherapeutic drugs with heightened efficiency, lowered toxicity to avoid the worst effects of chemotherapy, lower trauma to the patients, and not causing brain damage or weakening blood cells. The technology has been specifically developed for two of the most common types of leukemia (acute myeloid leukemia AML and relapsed acute lymphoblastic leukemia ALL) for which the preferred treatment is cytarabine-a highly toxic drug with severe side effects including cerebellar toxicity and bone marrow suppression. However, the drug often cannot be used at all.
Leukemia cells depend on an amino acid called aspargine, but they cannot synthesize it themselves; as a result, they “steal” it where they can, that is from within the bloodstream. Biosight has set up a molecular structure that leukemia cells recognize as being associated with aspargine, which they need, but instead they fill it with Astrabine, which kills them. Using this Trojan horse trick, they are able to destroy the cancerous cells while preserving the healthy ones. For another specific cancer, the problem would be to find the amino acid that this other cancer is ‘allergic’ to, and packaging it in a structure that the cancer cell thinks contains material that strengthens it, while in reality it contains material that destroys it. The technology could become very important in the field of cancer treatment and could really be the cure for cancer. Astrabine could bring real hope to many patients and an answer to unmet needs in the treatment of hematologic malignancies.
Researchers involved in a national effort to develop cancer treatments that harness nanotechnology are recommending pivotal changes in the field because experiments with laboratory animals and efforts based on current assumptions about drug delivery have largely failed to translate into successful clinical results. The assessment was advanced in a perspective paper titled “Targeting the Tumor Environment”, which appeared in the National Cancer Institute's “Cancer Nanotechnology Plan 2015”.
Researchers are trying to perfect "targeted delivery" methods using various agents, including an assortment of tiny nanometer-size structures, to selectively attack tumor tissue. However, the current direction of research has brought only limited progress. The bottom line is that so far there are only a few successful nanoparticle formulations approved and clinically used. Research with laboratory mice has rarely translated into successful clinical results in humans, suggesting that a more effective approach might be to concentrate on research using in vitro experiments that mimic human physiology. For example, one new system under development, called a “tumor-microenvironment-on-chip (T-MOC)” device, could allow researchers to study the complex environment surrounding tumors and the barriers that prevent the targeted delivery of therapeutic agents. One approach has been to design nanoparticles small enough to pass through pores in blood vessels surrounding tumors but too large to pass though the pores of vessels in healthy tissue. The endothelial cells that make up healthy blood vessels are well organized with tight junctions between them. However, the endothelial cells in blood vessels around tumors are irregular and misshapen, with loose gaps between the cells (Figure 4).
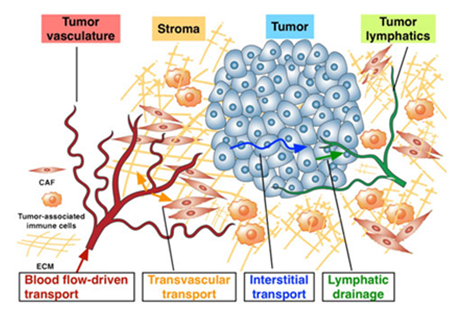
Figure 4 The Complex microenvironment of tumors presents a challenge in developing effective anticancer treatments that attempt to Harness Nanotechnology. (From Bumso Hau, Kinam Park and Murray Korc).
Treatments that Attempt to Harness Nanotechnology. (From Bumso Hau, Kinam Park and Murray Korc)
The approach could help drug makers solve a daunting obstacle: even if drugs are delivered to areas near the target tumor cells, the treatment still is hindered by the complex microenvironment of tumors. It is now known that merely killing the tumor cell would not cure the cancer because the complex environment (non-cancerous cells, blood vessel structure) supports the cancer cells. An "extracellular matrix" near tumors includes dense collagen bundles and a variety of enzymes, growth factors and cells such as, for example, surrounding pancreatic tumors in a "stromal compartment" containing a mixture of cells called stromal cells, activated cancer-associated fibroblasts, and inflammatory immune cells. The stromal tissue is much bigger than the tumor itself. In addition, a compound called hyaluronic acid in this stromal layer increases the toughness of the tumor microenvironment tissue, making it difficult for nanoparticles and drugs to penetrate.
Another challenge is to develop water-soluble drugs to effectively deliver medicines. Indeed, cancer drugs need to be aqueous because the body resorbs them better, but a lot of the current chemotherapy drugs have low solubility and usually need different types of solvents to increase their solubility. The T-MOC approach offers some hope of learning how to design more effective cancer treatments. Recent advances in tissue engineering and microfluidic technologies present an opportunity to realize in vitro platforms as alternatives to animal testing. However, such a major shift in research focus could play a role in developing personalized medicine (or precision medicine) tailored to a particular type of cancer and specific patients. More effective treatment might require various "priming agents" in combination with several drugs to be administered simultaneously or sequentially. Moreover, since small animal data have not been good predictors of clinical outcome, it is essential to develop in vitro test methods that can represent the microenvironment of human tumors.
The following is only an incomplete summary of advances in nanotechnology:
None.
Author declares there are no conflicts of interest.

©2016 Fymat. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.