Journal of
eISSN: 2373-633X


Case Report Volume 13 Issue 2
1Radiology Resident, Universidad del Norte, Colombia
2Neurosurgery, Neurodinamia SA, Colombia
3Interventional Neuroradiology, Neurodinamia SA, Colombia
Correspondence: Jennifer Richardson Maturana, Radiology Resident, Universidad del Norte. Km 5 via Puerto Colombia, Tel +57 3005351802
Received: February 24, 2022 | Published: March 24, 2022
Citation: Maturana JR, Perez RA, Manjarrez GDR, et al. Mixed vascular brain malformation: case presentation. J Cancer Prev & Curr Res. 2022;13(2):41-43. DOI: 10.15406/jcpcr.2022.13.00484
Developmental venous abnormality (DVA) is the most common benign intracranial vascular malformation, however, it is rare to see it associated with other malformations. It is generally asymptomatic and therefore an incidental finding on images. We present the case of a patient with headache and DVA with another associated malformation. We will review the clinical importance of the imaging identification of this entity for its treatment.
Keywords: central nervous system, venous angioma, cavernous angioma, complications
Developmental venous anomaly (DVA) is the most common benign intracranial vascular malformation. Its exact etiology is unknown, however, its nature is considered to correspond to a variation in the cerebral venous angioarchitecture from the embryonic stage.1 The medullary veins adopt a radial anatomical position that inverts the venous drainage of the cerebral or cerebellar parenchymal territory in which they are found to converge in a single drainage vein,2 which has been called the “jellyfish head” sign.3 It is generally an incidental finding since patients are usually asymptomatic. We present the case of a young patient with recurrent headache in whom a DVA associated with a cavernoma was identified by imaging. Neurological physical examination and vital signs are normal.
A 16-year-old female patient, with no history, who presented recurrent oppressive headache with occipital predominance that worsened, for which she consulted the emergency room.
Neurological physical examination and vital signs were normal. A simple skup CT was done showing a mass occupying the cerebellar vermis. Therefore, a contrast-enhanced MRI is performed in which the mass is characterized by having a "popcorn" configuration, with central hyperintensity on T1 and T2 with hypointense halo in all sequences (Figure 1) more evident in gradient echo which is compatible with a cavernoma (Figure 2). It is noteworthy that in the contrasted T1 sequence an DVA is found adjacent to the cavernoma with a subependymal drainage vein (Figure 3).
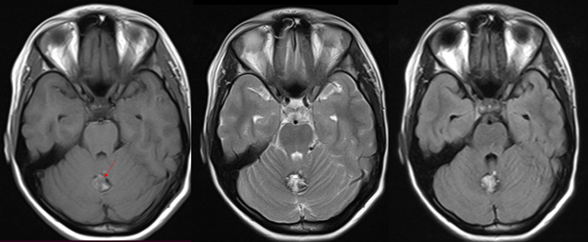
Figure 1 Axial images with information on T1, T2 and FLAIR, showing a “popcorn” mass at the level of the cerebellar vermis (arrow) isointense to the cerebellar parenchyma on T1, and hyperintense on T2 and FLAIR with a hypointense halo by haemosiderin which is compatible with cavernoma.

Figure 2 Axial images with information on DWI, ADC and gradient echo of the cavernoma described in Figure 1. There is no diffusion restriction, with "blooming" on gradient echo.

Figure 3 Axial, sagittal and coronal images with T1 information with contrast (gadolinium). In the vermian region, enhancement of veins in a radial arrangement that converge to a more prominent vein in the direction of the straight sinus related to venous angioma (arrow) is observed.
Given the recurrent symptomatology of the headache reported by the patient and the cavernoma found in the images, cerebral digital subtraction angiography (DSA) is recommended, in the arterial phase with no pathological findings (Figure 4) and the DVA in the territory evident in the venous phase. Right posterosuperior cerebellar with confluent drainage vein towards the straight sinus (Figure 5).

Figure 5 Digital subtraction angiography of the left vertebral artery in the venous phase showing the DVA with the “jellyfish head” sign on the right side (arrow) with a vein draining to the straight sinus.
The Neurodynamics multidisciplinary team recommends follow-up of the ADL, and consider symptomatic or surgical management of the cavernoma according to the assessment and criteria of your treating physician.
Developmental venous anomaly (DVA) is the most common cerebral vascular malformation, accounting for 55% of all these lesions.1 It was first described by Lasjaunias and Burrows,4 it is also known as venous angioma, cerebral venous malformation and cerebral venous medullary malformation, this being the terminology that best describes its nature. Its etiology is unknown, however, it is recognized that from the embryonic period there is an alteration in the development of the medullary veins in which they acquire a radial configuration and drain into a single dilated vein that can communicate with the superficial cerebral drainage system or deep.2
Clinically they are asymptomatic in most cases, however, some patients may present with symptoms related to intracranial hemorrhage, ischemic stroke and epilepsy, which present as complications in 0.15% of cases (hydrocephalus, neurovascular compression, increased the volume of the venous drainage or decrease in its output).1,5
ADLs are an incidental finding, with a prevalence of 2.5-9% on contrast-enhanced magnetic resonance imaging (MRI) and digital subtraction angiography (DSA),6 in which the jellyfish head sign or palm tree (figures 3 and 5) that represents the abnormal medullary veins draining into a single dilated collecting vein, these in turn drain into a dural sinus or an ependymal vein. The most common locations of ADLs are the frontoparietal region (36-64%) and the cerebellum (14-27%).7
If the drainage vein is large, it can be seen on simple tomography (CT) and confirmed on contrast-enhanced CT, with the same imaging characteristics described.4 Some associated dystrophic calcifications can be observed in 9.6% of cases, characteristically located in the basal ganglia and unilateral.8
Regarding the magnetic resonance imaging (MRI) sequences, the ADL is better observed in the SWI sequences since these are more sensitive to low venous flow, adequately delimiting the anatomy of the ADL,6 which can also be characterized very well. well with contrast-enhanced T1 (Figure 3).
Digital subtraction angiography (DSA) is obtained in order to confirm the CT and MR findings, and to rule out other abnormalities such as associated arteriovenous malformations.4 Likewise, it accurately shows in real mode the drainage towards the dural sinuses. It is important to know that DVA will not be observed in the arterial and capillary phase (Figure 4), therefore, the standard image of this modality will be the venous phase (Figure 5).
ADLs are solitary in 75% of cases. Only about 20% can be associated with other cavernous malformations, as in our case, and are then called mixed vascular malformations.9 It can also be associated with venous malformations of the head and neck or cortical dysplasia.
Cavernomas are the third most common cerebral vascular malformation. It has been called in many ways: cerebral cavernous malformation, hemangioma, etc. However, the most recent nomenclature of the ISSVA classification of vascular anomalies,10 considers that the term cavernoma is the most appropriate for clinical interpretation.
Cavernomas generally occur in asymptomatic patients between 40-60 years of age, and therefore are an incidental finding. However, they can be symptomatic when bleeding, manifesting with headache, seizures or focal neurological deficit. Multiple brain lesions could be related to familial multiple cavernous malformation síndrome.11
The MR cavernoma will have the classic hyperintense “popcorn” image on T1, T2 and FLAIR in its center (Figure 1), without restriction on DWI-ADC sequences and with a halo of haemosiderin visible on all sequences, more evident in T2*, with “blooming” artifact in gradient echo (Figure 2).4,6 Isolated ADLs do not require treatment, since obliterating the only drainage system in the territory where they are located can generate a venous infarction.4 If it is part of a mixed vascular malformation, the treatment will be based on the other component, as in our case, the surgical management or follow-up of the cavernoma. That is why it is important for us as radiologists to identify and describe the drainage system that the AVM has.
Venous angiomas represent one of the most frequent radiological findings in our practice, it is essential that we identify if they are related to other malformations since these may be susceptible to neurosurgical management in the event of complications, which directly impacts the morbidity and mortality of patients.
No funding of any kind has been received for the completion of this article.
None.
The authors declare no conflict of interest.

©2022 Maturana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.