Journal of
eISSN: 2373-633X


Research Article Volume 6 Issue 4
King Khalid Eye Specialist Hospital, Saudi Arabia
Correspondence: Rajiv Khandekar, Research department, King Khalid Eye Specialist Hospital, POB: 7191, Aruba Road, Riyadh, Pin: 11462, Saudi Arabia,, Tel 9.66115E+11, Fax 9664829311
Received: November 27, 2016 | Published: December 21, 2016
Citation: Elkhamary SM, Sallam AA, Al Kahtani E, et al. Measurement of bony orbital volume with computed tomography (CT) in healthy eyes of Saudi children. J Cancer Prev Curr Res. 2016;6(4):503-507. DOI: 10.15406/jcpcr.2016.06.00215
Purpose: To provide age and gender-specific reference values of bony orbital volume in normal healthy eyes of Saudi Arabian children.
Methods: A retrospective review of health data was performed between October 2014 to May 2015. Healthy eyes of children aged 15years or younger without orbital disease were included. Their computed tomography (CT) scans were reviewed. Orbital bony cavity volume (OV) was calculated by the 3D-semi-automated volumetric methods. Variation of OV by age and gender was analyzed.
Results: CT scans of 374 children (197 males, 177 females) were reviewed. The mean OV of healthy Saudi children was 19.1mm3 95% confidence interval (CI):18.7–19.5. OV was statistically significantly larger in males than females (difference of mean=1.23mm3 (95% CI: 0.7–1.8) P<0.0001. There was a positive, statistically significantly association of OV to age (P<0.0001).
Conclusion: OV of normal healthy eyes of Saudi Arabian children statistically significantly differed between gender and with age. The 3D volumetric method presents a reliable method for orbital volumetry. The rapid phase in orbital growth in Saudi males and females seems to end at 15years and 11years old respectively.
Advances in Knowledge: Orbital volume (OV) of 374 Saudi children measured by semiautomatic method (CT scan) was 19.1mm3. The OV of boys was larger than of girls. With increasing age, the OV increased but in different proportions in boys and girls.
Keywords: orbit, pediatric ophthalmology, computer assisted tomography (CAT), orbital volumetry
The bony orbit surrounding the eye is pyramidal with a rectangular base. The orbital volume (OV) of an adult is approximately 30mm3.1 However, measurement of OV can be inconsistent due to the complicated anatomical structure of the bony orbit including the irregular inner border, holes and fissures. Computed tomography (CT) provides excellent image quality of bony landmarks making it a popular imaging technique for viewing orbital pathology. Additionally, CT is accurate and reproducible, due to the 3-dimensional image capabilities.2 Change in OV is a dynamic process. For example, short and long-term changes occur due to aging of soft tissue and bony structures resulting changes in shape, size, and volume of the bony orbit.3,4 Variation in OV has been documented in Asian and Caucasian populations.5 Changes to OV had been documented in ethnic groups. For example, OV decreased in Arab adults after enucleation.6‒8 There is relative paucity of normative OV data in many populations worldwide.2 To the best of our knowledge, normative OV data for the Arab population is not available in the literature. In this study, we measure the dimensions and OV of the bony orbit in Saudi children.
The Institutional Review Board of the King Khalid Eye Specialist Hospital (KKESH) approved this study. Clinical records were de-linked from the personal identifiers prior to analysis. A retrospective chart review was performed between January and June 2015. Saudi children aged 15years or younger, undergoing orbital CT scans between January 2013 and September 2014 with unilateral ocular pathology were included in the study. Children with bilateral orbital pathology, congenital craniofacial malformations, previous orbital or eye surgery, systemic malignant disease, recent trauma, silent sinus syndrome, neoplasm involving the bony orbit or the orbital tissues and any other disorders known to influence the orbit and/or orbital contents or those with gantry tilt on CT scanning were excluded from the study.
To estimate a representative sample of Saudi children, we assumed that variation in volume by age and gender was 10% and our study was sensitive enough to identify the variation ranging between 7.5% to 12.5%. To compensate for the patient dropout, we increased the sample by 10%. Thus the final sample size was at least 374 eyes/orbits. CT data were acquired using Discovery 750 HD 64-slice scan (GE Healthcare, Milwaukee, WI, USA). The scanning protocol included 0.6-mm axial, non-overlapping contiguous sections for the orbits. This was achieved by tilting the patient’s head parallel to the Frankfurt plane. Bone and soft-tissue algorithm reconstructions were available for review. OV measurements were performed at the same sitting by a senior neuro-radiologist (SE) and digital radiographic technician (AS). For image processing and analysis, all CT images were exported in DICOM format into the Advantage Windows, version 4.6 (GE Healthcare, Milwaukee, WI, USA). To reduce measurement errors, the rules for orbital volume calculation and anatomical landmarks were standardized. OV was measured twice and then averaged. All measurements were performed at a constant window level and width settings.
For every child, OV was calculated by a 3D-assisted semi-automated volumetric methodology using the 3D volume rendering tool (VR).2,3 This method includes a mixture of manual segmentation performed using seed-growing algorithms with contour constraining, using the three-dimensional reconstructed images then rendering software volume calculation with a graphic representation of the 3D configuration of the orbital cavities (Figure 1). This 3D Volume VR tool is available on Advantage Window. The program displays axial, coronal and sagittal images on the same display screen. The measurement area of the orbit ranged from the lacrimal fossa to the optic canal. A straight line through the lateral and medial orbital rims defined the anterior border of the orbit. The posterior limit was set at the opening of the optic foramen into the orbit. On the axial slice, the ‘‘VR tools, add segment, auto select tool, any structure, was selected and the ‘‘threshold’’ was set between 0 and 160 with the window width 300, window length 35 HU (Hounsfield unit), then the orbital contents were traced and marked using a digital mouse and by left-clicking, we started at the mid-orbit with the area of interest colored red using autoselection for each slice. Then the slice was scrolled stepwise in a cranio-caudal direction until all the orbital contents of interest were colored. Next, we moved from axial to the sagittal view and then a coronal slice and repeated the same procedure to confirm the most anterior, posterior, superior, and inferior limits using auto-location on a sagittal-plane view. The optic canal, soft tissue and portions of the globe protruding out of the orbital rim were excluded from volume calculation. During tracing, the observer had to work with all three-images as stacks as segmentation performed in one image automatically appeared in the other two due to the three-dimensional visual control of the measurements at any moment during the measurement that allowed the observer to visually check the result of the calculations instead of outlining the structures of interest in every slice.
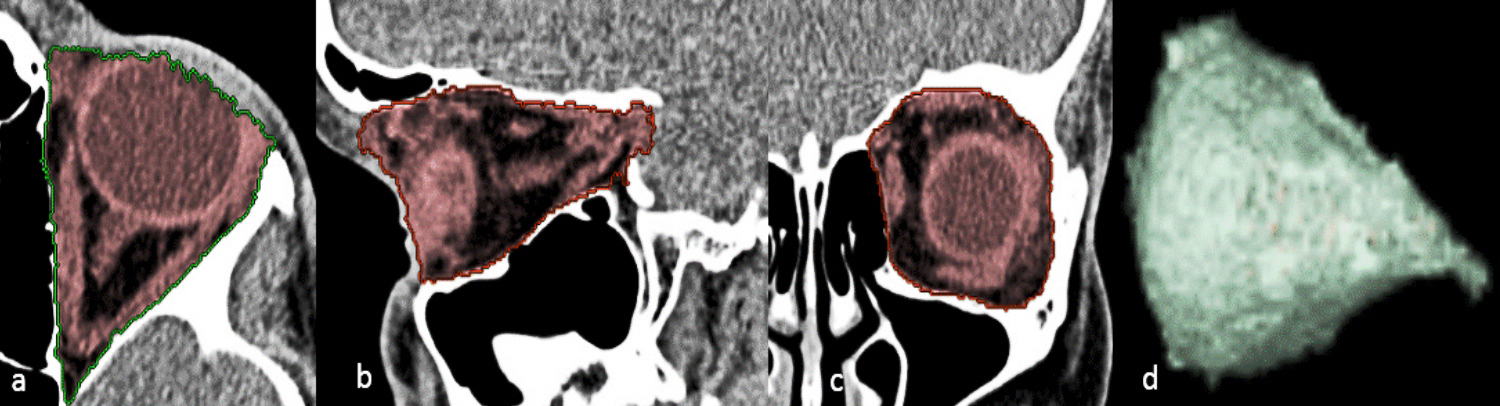
Figure 1 Computed tomography showing the process of segmentation for OV measurement using 3D -semi-automated volumetric methodology. The highlighted segment of tissue content of the orbit with the boundaries of the bony orbital cavity is denoted by green and the traced orbital content is marked as red and measured in (a, axial b, sagittal c, and coronal images with d, Generated conical shape 3D-reconstruction of the bony orbital contents.
The erase structure key was to edit and deselect erroneous areas. After defining the region of interest (ROIs) on consecutive slices, the traced area was automatically colored red and the bony orbital border was marked by a green line. To see the volume of the marked orbital content, ‘‘Display Tools’’ ‘‘measure volume’’ and ‘‘Apply’’ Keys were used. In addition, a graphic representation of the 3D conical shape of the orbital cavities was constructed and saved in full STL format. Data were collected on patient demographics, orbital height, diameter of the base and OV on an Excel spreadsheet (Microsoft Corp., Redmond, WA, USA). Data were analyzed with Statistical Package for Social Studies (SPSS 22) (IBM Corp., New York, NY, USA). The data were tested for a normal distribution. The mean and standard deviation and range were calculated. The variation of volume by age was analyzed. Due to the skewed distribution of age (in years), the log values of age were calculated to determine normalized mean and standard deviation of age. The difference of means and 95% confidence interval (CI) were used to evaluate the variation of OV by gender, location (zone) based on residence in Saudi Arabia. Statistical significance was indicated by two-sided P<0.05.
This study included orbit measurements of 374 children. There were 197 (53%) males and 177 (47%) females. The mean age of the participants was 6.4±2.1years (range; 2months to 15.4years). The right orbits of 174 (46.8%) children and left orbits of 200 (53.2%) children were included in this study. The OV using semi-automated method is presented in Table 1. The OV was 19.1 mm3 (95% CI: 18.7-19.5) with the 3D-semi-automated method. OV in males was statistically significantly larger than females (difference of mean=1.25 mm3 (95% CI of difference of mean: 0.71–1.8); P<0.001. The OV increased with age. The variation of OV was significantly associated to the age of the participant (P<0.001). The linear relation of age and OV in males and females is presented in Figure 2.
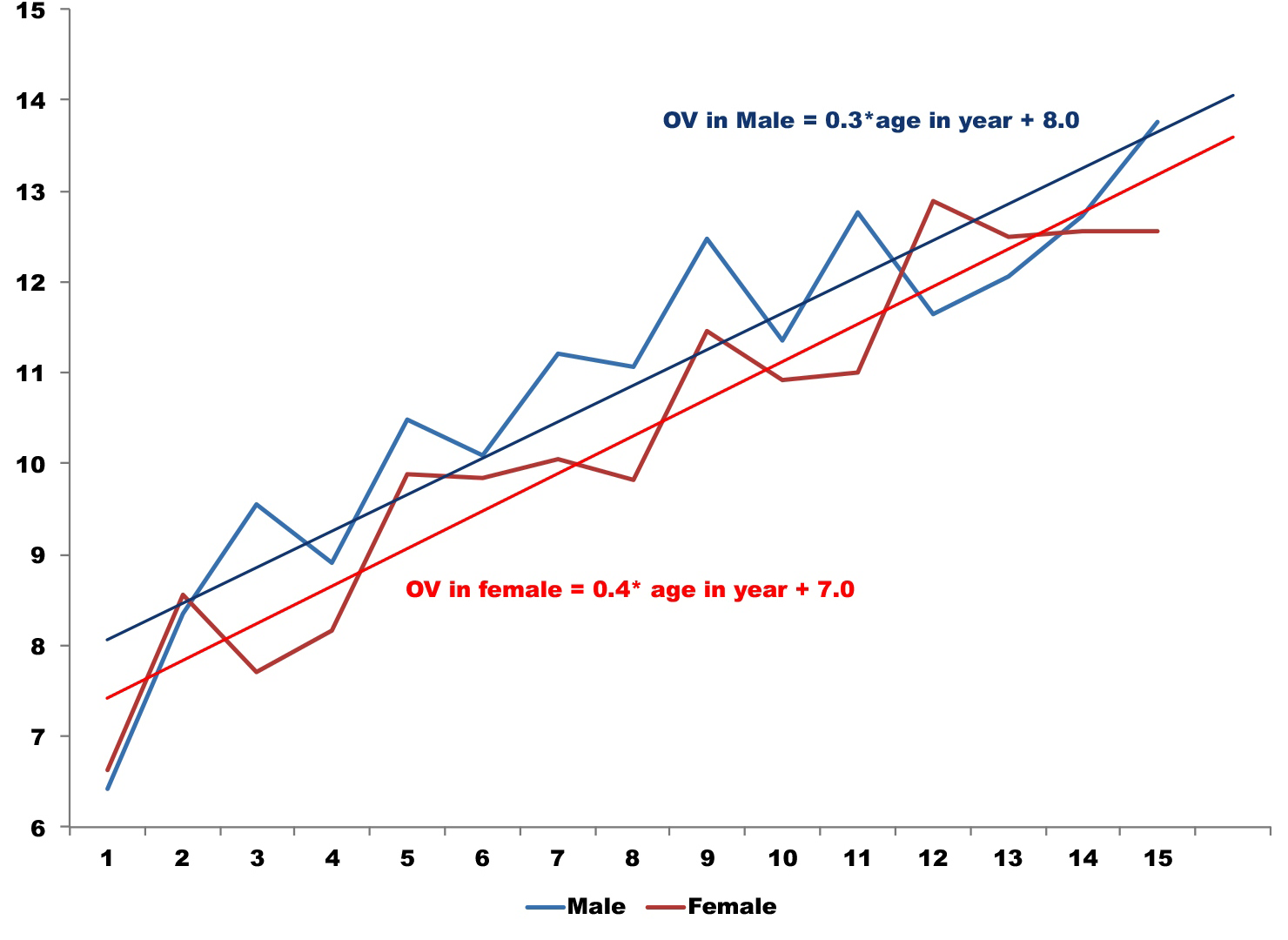
Figure 2 Linear relation between age-sex and orbital volume.
The age in year is dipicted in X axis while orbital volume in mm3 is plotted in Y axis of the graph.
The dotted lines (blue for male and red for females) are the volumes noted. The solid line is the trend line with equation.
Non pathologic eyes of Saudi children |
Number |
3D-semi-automated volumetric methodology (cm3) |
Statistical validation |
|||
Mean |
SDV |
95% CI |
P value |
|||
Total |
374 |
19.1 |
3.9 |
18.7 – 19.5 |
||
Gender |
Male |
197 |
20 |
3.57 |
19.5 – 20.5 |
<0.001 |
Female |
177 |
18.1 |
3.95 |
17.5-18.7 |
||
Age in years |
1 |
23 |
12.6 |
3.24 |
11.3 – 14.0 |
<0.001 |
2 |
20 |
17.4 |
4.42 |
15.5 – 19.3 |
||
3 |
26 |
16.3 |
3.88 |
14.8 – 17.8 |
||
4 |
30 |
15.6 |
3.43 |
14.3 – 16.8 |
||
5 |
26 |
19.2 |
2.73 |
18.1 – 20.3 |
||
6 |
19 |
19.1 |
2.2 |
18.0 – 20.1 |
||
7 |
24 |
20 |
2.1 |
19.1 – 20.9 |
||
8 |
28 |
19.2 |
3.1 |
18.0 -20.4 |
||
9 |
35 |
20.3 |
2.41 |
19.5 – 21.1 |
||
10 |
15 |
21 |
2.58 |
19.6 – 22.4 |
||
11 |
18 |
21.3 |
3 |
19.8 – 22.8 |
||
12 |
19 |
21.5 |
1.9 |
20.6 – 22.4 |
||
13 |
15 |
21.9 |
2 |
20.8 – 23.0 |
||
14 |
27 |
21.6 |
2.54 |
20.6 – 22.6 |
||
15 |
23 |
22.2 |
2 |
21.3 -23.1 |
||
15+ |
14 |
20.3 |
3.9 |
18.0 – 22.6 |
||
Table 1 Orbital volume of normal Saudi children
The mean height of the orbit was 36±3.8mm. The variation in height in males and females was similar (P=0.03). The variation in height by age-group was statistically significant (P <0.001). The mean width of the orbital opening (base) was 33.2±3.3mm. The variation in width of base of the orbit by gender was statistically significant (P<0.001). The variation in width of base of orbit by age-group was statistically significant (P<0.001). We compared outcomes of our study with other studies selected from the peer review literature (Table 2). There were racial and methodology differences in between studies. The OV measurement with the 3D-semi-automated method had less variability.
|
Author |
Orbital Volume (ml) |
Ethnicity |
Age years |
Imaging Modality |
Methods Notes |
|
|
Male Female |
||||||
|
Bentely et al.,10 |
First months 15 |
13 |
0-15 years |
MRI |
||
|
15 years 27 |
25 |
|||||
|
Forbes et al.,12 |
upper limit of 30.1 cm3 |
- |
Western7 |
Adult |
||
|
Chau et al.,14 |
22.2±1.4 |
19.8±2.2 |
Hong Kong Chinese |
MRI |
OSIRIS software |
|
|
Chen et al.,16 |
Method 2: |
19.1±1.5 |
Chinese |
Different age group |
CT |
5mm CT slice thickness/distance protocol |
|
Our study |
Method Two: |
16.0±0.9 |
Saudi |
0-15 |
CT |
3D-semi-automated volumetric methodology |
Table 2 Comparison of orbital volume in different studies
Our study provided reference values of OV for healthy Saudi children. It demonstrated higher OV values in male compared to female children. The OV measured by the computer assisted volumetric method is a reliable measure. Previous studies have mainly focused on bony orbit after enucleation, after traumatic anophthalmos and had used a variety of measurement techniques.9 Normative data on OV of Asian and Western races are available.4,10,11 However, few studies have focused on children. Comparison between studies should be performed with caution due to the differing measurement techniques and patient populations. The data obtained from the present study can be used for comparison to the OV obtained from diseased orbits. This comparison with help in monitoring conditions that affect development of the orbit and planning orbital reconstruction surgery in Arab children.
The OV of Saudi children measured in the present study was 19.1±3.9 mm3 by the semi-automated method. The OV in our study was larger than Japanese males and females.12 Forbes et al.,13 used CT to estimate OV in subject from the United States and found much larger volumes for the normal bony orbit. These values are larger as they were from an adult population and included all of the soft tissues surrounding the eye. In contrast, OV was smaller in children from Far Eastern populations compared to the Arab children in our study.1,14‒16 Thus skull and bony orbit size seems to be influenced by race and therefore normative data for one race of children may not accurate for a different race.
The OV in Saudi male children was significantly larger than OV in Saudi female children. Similar gender variation was noted in other studie.1,5,12‒18 Bentley et al.,10 used MRI to measure OV of mainly Caucasians and found that OV was larger in British children compared to Saudi children. In MRI, the bones of the orbital rim are noted as a dark signal. In contrast, CT scan (as used in our study) imaging of bones appears as bright dense signals. Hence, the outlines of the CT image will be smaller but more accurate. This explains the variation noted in the our study and study by Bentley et al. Futura et al.,12 reported larger OV in males compared to females. In contrast, Forbes et al.,13 found no statistically significant difference between genders. The orbital size correlates well with the bone size of the skeleton for the entire body because of the growth pattern and skull length.19,20 This observation explains the gender variation of OV.
In Saudi children, the OV increased from 12.6mm3 in the 1st year to 22.2mm3 at 15years of age. Chau et al.,15 measured OV using MRI of Chinese subjects. The mean OV of children in Chau et al.,15 study was lower than our study. The OV increased from 12.4cm3 in <1year old to 15.3cm3 in 1 to 3years old children. By 3years of age, OV reached 60% and 78% of adult size by 3 and 10years of age. The pattern of OV growth by age in both these studies is similar. We noted that age and gender had influenced changes in OV in Saudi children. Throughout childhood, OV were larger in males than females. However, the growth pattern by age was similar in both genders. The difference of OV growth among male aged <5years was significantly greater than females of same ages (P<0.001) implying that major OV growth takes place in early in the lifetime among Saudi children.
There was no significant difference in OV between genders younger than 16years old in our study. In contrast, Chen et al.,16 noted a linear correlation between the age and OV. They reported that OV increased rapidly before 20 years of age and the mean OV reached 95% of the adult OV at 13 years in males and 17years in females.16 Seventy percent of the increase in OV occurred in Saudi children by the time the child reached 5years of age. Such linear increases in OV growth has been noted by other researchers.7,10,12,15,21 By the time a child has reached 5years of age, the OV for both right and left sides reached, on average, 77% of the volume seen at 15years of age in both genders in our study. Furuta et al.,12 found a strong correlation between age and OV with an almost identical approximate equation for both sexes until 12years of age. The OV increased significantly in males between 12 and 15years of age whereas in females it increased rapidly between 6 and 11years of age.12 Thus, more than 95% of the growth of the adult orbit is completed by the first half of the teenage years. Forbes et al.,13 found that OV increased rapidly until age 14.9years in males and until 10.9years in females. Any reconstructive surgery of the orbit during these teenage years should note the variation in growth pattern between genders.
The volume of the right and left orbits in our study did not differ. The differences between the two sides were similar between genders in another study10 since, facial asymmetry was an exclusion criteria in the present study, we did not expect variation in OV based on the side of the orbit involved. We found, the variation in height and width by age group was statistically significant (P<0.001) but not gender specific. This concurs with the findings of Forbes et al.,13 This observation suggests that OV can also be measured reliably one-dimensionally. The difficulty in manually evaluating the OV is mainly related to the complicated anatomical structure of the bony orbit. The semi-automated OV accurately measures each slice of image. This could be the reason for accurate and larger OV in semi-automated method. The segmentation in semi-automated methodology is based on the pixel measurement, which is limited by the difference in bony landmarks using a semi-quantitative assessment. Image orientation is a key factor for accurate volume analysis.
The 3D-semi-automated method used in the present study is a bit complex since it includes segmentation on thin slice reconstructed three-dimensional images. A constant window level and width, with a manual trace method with contour constraining is required and OV calculated from the sum of each traced area. In 3D-semi-automated method, several areas of the orbit had to be avoided because they included soft tissue that is outside the plane of the boundaries of the orbits. Another limitation is the difficulty in defining the anterior border of the bony orbit. 20‒25 Additionally, more anatomical and computer knowledge and time are required for the 3D-semi-automated method. Therefore, 3D-volume rendering and region-of-interest (ROI) for volume computations provides information that could complement available information based on traditional two-dimensional images. A larger number of subjects can be assessed in this method and quantify normal orbital anthropometry across individuals according to gender, ages and ethnicities. There are some limitations to our study. We studied children who had pathology in the fellow eye. Although clinically the second eye and orbit were normal, pathology in fellow eye in some children may have influenced OV measurements. Further studies on OV volume of children with bilateral healthy eyes are recommended.
OV of normal healthy eyes of Saudi Arabian children differed by gender and age. The 3D volumetric method is a reliable tool for measuring OV. The rapid phase in orbital growth in Saudi males and females seem to end at 15years and 11years old respectively.
Sahar Al Khamary: Planning, interpretation of data and finalization of manuscript.
Abdullah Ali Sallam: Planning, data collection and drafting manuscript.
Eman Al Kahtani: interpretation of data and drafting manuscript.
Ches Surou: literature review, field work, data management, draft manuscript.
Rajiv Khandekar: Planning, data analysis, interpretation of data and drafting manuscript.
None.
Authors declare there are no conflicts of interest.

©2016 Elkhamary, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.