Journal of
eISSN: 2373-633X


Case Report Volume 4 Issue 2
Department of Radiology, DM Wayanad Institute of Medical Sciences, India
Correspondence: Krishna Kumar M, Associate Professor & HOD, Department of Radiology, DM Wayanad Institute of Medical Sciences, Naseera Nagar, Meppadi PO, Wayanad, Kerala, Tel 9.18E+11, Fax 914936-287287
Received: December 20, 2015 | Published: February 3, 2016
Citation: Krishna KM. Mdct - evaluation of cardiac manifestations of kawasaki disease. J Cancer Prev Curr Res. 2016;4(2):50-52. DOI: 10.15406/jcpcr.2016.04.00113
Kawasaki disease (KD) is an acute self-limited vasculitis of childhood that is characterized by fever, bilateral non exudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy. Coronary artery aneurysms or ectasia develop in 15% to 25% of untreated children and may lead to ischemic heart disease or sudden death. We report a 3 years old female presented with high and persistent fever for 1- 4 weeks & pericardial effusion, not very responsive to treatment with paracetamol or antibiotics and diagnosed as Kawasaki disease, referred for Multi detector Computed Tomography (MDCT) evaluation.
Keywords: coronary aneurysm, coronary artery ectasia, infantile polyarteritis nodosa, kawasaki’s disease, kawasaki’s syndrome, mucocutaneous lymph node syndrome, multi detector computed tomography
KD, kawasaki disease; MDCT, multi detector computed tomography; MI, myocardial infarction; LMCA, left main coronary artery; LAD, left anterior descending artery; RCA, right coronary artery; AHA, American heart association; JCS, Japanese circulation society
Kawasaki disease (KD) is an acute, self-limited vasculitis of unknown etiology that occurs predominantly in infants and young children. First described in Japan in l967 by Tomisaku Kawasaki, the disease is now known to occur in both endemic and community-wide epidemic forms in the Americas, Europe, and Asia in children of all races.1 KD is characterized by fever, bilateral non exudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy. Coronary artery aneurysms or ectasia develop in 15% to 25% of untreated children with the disease and may lead to myocardial infarction (MI), sudden death, or ischemic heart disease.2,3 We evaluated the cardiac manifestations of Kawasaki disease by MDCT in a 3 year old female child.
We report a 3 years old female child, presented with high and persistent fever for 1-4 weeks, not very responsive to treatment with paracetamol or antibiotics. Her clinical examination showed - redness of tongue, swolllen lips, erythema of the palms, generalised joint pains, throat pain (pharyngitis) & rashes in the body. Laboratory findings-revealed Thrombocytosis (platelets 7.5lakhs) & Leucopenia (WBC 4000). Echocardiography revealed moderate Pericardial effusion and suspicious coronary dilatation. The child was diagnosed as a case of Kawasaki disease (KD) and referred for Multi detector Computed Tomography (MDCT) evaluation to rule out coronary abnormalities. The patient underwent Cardiac CT on a 256 slice MDCT scanner (Brilliance iCT 256 slice, Philips Health care). The images were processed by iDose4 reconstruction using Philips EBW 4.5 workstation.
Cardiac CT protocol
270msec rotation, spiral mode, retrospective ECG gating, collimation 128x0.625mm. Average heart rate during the procedure was 136beats/minute. Axial thin CECT sections taken for the cardia followed by 3D, MIP, Short axis and Long axis reconstruction 2 Chamber, 3 chamber, 4 chamber views of cardia are studied.
IV contrast
Omnipaque (iohexol) 350mg/ml. 20ml at 5ml/sec followed by saline flush. No Premedication was given before the procedure. Discrete, coronary aneurysms (defined as segmental dilation between 1.50- to 2.0-fold larger than maximal normal diameter of the respective vessel)4 was documented in the distal Left main coronary artery (LMCA), proximal Left anterior descending artery (LAD) and proximal Right coronary artery (RCA). Moderate pericardial effusion (11-21mm) was noted (Figure 1). Coronary artery ectasia (defined as diffuse, uniform luminal dilation between 1.50- to 2.0-fold larger than maximal normal diameter of the respective vessel)4 was documented in the proximal left circumflex coronary artery (Diameter 3.5mm) (Figure 2). The fusiform aneurysm of distal LMCA & LAD (Figure 3) measured 12.6 mm in diameter and about 31 mm in length. No evidence of any intra luminal thrombus noted. The fusiform proximal RCA aneurysm (Figure 4 & Figure 5) measured 13.1mm in diameter and 59 mm in length. Significant stenosis was not detected in any of them. The mitral valve, tricuspid valve, aortic valve and pulmonary valves were normal. Main Pulmonary, Right pulmonary & Left pulmonary arteries, Aortic root & ascending aorta were normal. Cardiac chambers were within normal limits. In view of the persistent fever, typical clinical findings of KD, coronary artery dilatation and pericardial effusion, confirmed the diagnosis of Kawasaki disease. The patient was treated with intravenous gamma-globulin (IVIG), corticosteroids, aspirin & anticoagulants.
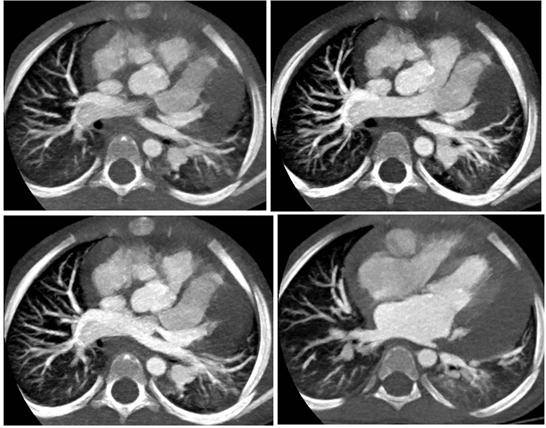
Figure 1 Axial Cardiac CT of 3 years old female child presenting with 1-4 weeks fever, reveals moderate pericardial effusion with dilated RCA & LAD.
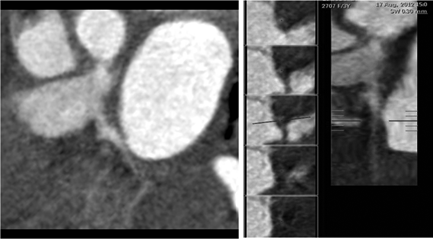
Figure 2 A) Curved planar reformatted image B) Straight vessel analysis shows focal Ectasia of proximal LCX.
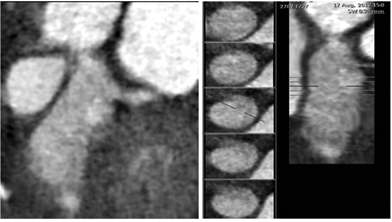
Figure 3 A) Curved planar reformatted image B) Straight vessel analysis reveals LAD fusiform aneurysm involving distal LMCA.
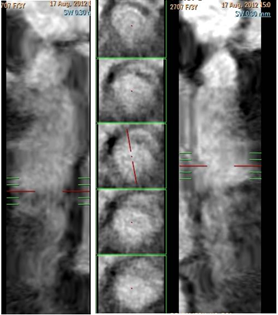
Figure 4 A) Curved planar reformatted image B) Straight vessel analysis shows RCA fusiform aneurysm. Note the decreased attenuation of its lumen, as compared with the proximal segment, a finding that is due to the slow flow within the aneurysm.

Figure 5 Double-oblique coronal maximum intensity projection images shows fusiform aneurysm of RCA, distal LMCA & LAD.
The patient was followed up for 3 months with echocardiography, which showed progressive resolution of coronary aneurysms. Long term follow up with MDCT was not done, as the patient was lost for follow up.
The etiology of Kawasaki disease (KD) remains unknown, although an infectious agent is strongly suspected based on clinical and epidemiologic features. A genetic predisposition is also likely, based on varying incidences among ethnic groups, with higher rates in Asians. KD is the leading cause of acquired heart disease among children in developed countries. KD is markedly more prevalent in Japan and in children of Japanese ancestry, with an annual incidence of 112 cases per 100 000 children less than 5 years old.5 Males are more commonly affected and most patients are less than 5 years of age. KD is a generalized systemic vasculitis involving blood vessels throughout the body. Arterial remodeling or revascularization may occur in KD with coronary arteritis. Progressive stenosis in the disease results from active remodeling with intimal proliferation and neoangiogenesis; the intima is markedly thickened and consists of linearly arranged micro vessels, a layer that is rich in smooth muscle cells, and fibrous layers. Several growth factors are prominently expressed at the inlet and outlet of aneurysms, where they are activated by high shear stress.6 There is no specific diagnostic assay for KD; therefore, diagnosis is based on clinical criteria, which include fever for at least five days and four or more of the five major clinical features (i.e., conjunctival injection, cervical lymphadenopathy, oral mucosal changes, polymorphous rash, and swelling or redness of the extremities), and exclusion of alternative diagnoses.3 In addition, classic KD can be diagnosed with three clinical features if coronary artery abnormalities are observed on echocardiography or coronary angiography.4 The disease typically follows three phases-acute (1-2weeks), subacute (2-8weeks) and convalescent (months-years). Cardiac sequelae are the most important manifestations of Kawasaki disease and include coronary artery dilatation, premature atherosclerosis and stenosis (4.7%), thrombosis, or occlusion with myocardial infarction (1.9%).2 Other cardiac complications include myocarditis, pericarditis, congestive cardiac failure, pericardial effusion, mitral or aortic insufficiency and arrhythmias. Cardiac manifestations are the leading cause of long term morbidity and mortality. Coronary artery aneurysms are defined as coronary artery segments that (a) have a diameter that exceeds the diameter of normal adjacent coronary segments or the diameter of the patient’s largest coronary vessel by 1.5 times and (b) involve less than 50% of the total length of the vessel . The term ectasia is used to describe diffuse dilatation (>1.5 times the normal diameter) of the coronary arteries that involves 50% or more of the length of the artery.7 The left main coronary artery is involved in 12% of the cases, the RCA in 3%, and both arteries in 8%.8 Recently, guidelines were published by the American Heart Association (AHA) to aid in the diagnosis and management of KD. Without treatment, coronary artery abnormalities develop in about 15 to 25 percent of patients with KD. Fortunately, with prompt therapy this percentage decreases to about 5 percent for any abnormality (including transient abnormalities) and 1 percent for giant coronary artery aneurysms. About 85 to 90 percent of patients respond promptly to initial therapy of IVIG and high dose aspirin; however, others have persistent or recurrent fever beyond 36 hours of therapy and require further treatment.9 Although low-dose aspirin is adequate for patients with mild Coronary disease (e.g., dilation; small, stable aneurysm), additional therapy such as antiplatelet agents and heparin may be required for patients with more severe disease because of the increased risk of thrombosis from the abnormal blood flow through coronary aneurysms.4 About one half of the coronary artery aneurysms associated with KD resolve by echocardiography and angiography within 1-2 years, particularly those that are smaller and fusiform.9
Regarding clinical management of patients with a history of KD, Two consensus guidelines have been published, one by the American Heart Association (AHA) in 2004, and one by the Japanese Circulation Society (JCS) in 2014.4 Coronary artery evaluation can be easily combined with the assessment of ischemia, infarction, ventricular dimensions, and function within the same procedure, for which cardiac (CMRI) has become the standard.10 A recent study in infants and young children with KD showed that low-dose, free-breathing prospective ECG triggered CT-angiography without the use of beta-blockers diagnosed all CAA detected with ultrasound plus the more distal aneurysms and dilatations.11 Apart from replacing invasive coronary angiography, in an early stage of follow-up, low-radiation dose CT-angiography can also be used at a later stage to check for the development of stenosis in patients with CAA. There are no specific recommendations for imaging modalities. Therefore, serial imaging and stress tests are necessary in patients with significant coronary artery abnormalities, and cardiac catheterization with angiography often is performed to better delineate the morphology once inflammation has resolved. Decisions about interventions for individual patients usually should be made in consultation with a cardiac surgeon and an experienced adult interventional cardiologist. Differential Diagnosis of KD include: Viral infections (eg, measles, adenovirus, enterovirus, Epstein-Barr virus), Scarlet fever, Staphylococcal scalded skin syndrome, Toxic shock syndrome, Bacterial cervical lymphadenitis, Drug hypersensitivity reactions, Stevens-Johnson syndrome, Juvenile rheumatoid arthritis, Rocky Mountain spotted fever, Leptospirosis and Mercury hypersensitivity reaction (acrodynia).4 The current study has the following limitations.
KD is the leading cause of acquired heart disease among children in developed countries. Coronary artery aneurysms or ectasia develop in 15% to 25% of untreated Children and treatment with IVIG in the acute phase of the disease reduces this risk to 5%. Cardiac ultrasound, invasive coronary catheterization, cardiac CTA and cardiac MRI are useful tools to detect, characterize and follow coronary abnormalities. A single comprehensive cardiac MDCT examination applied in KD, during subacute and convalescent phases may provide important diagnostic information on coronary anatomy, and thus may be helpful for therapeutic decision making.
None.
The authors declare that there is no conflict of interest.

©2016 Krishna. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.