Journal of
eISSN: 2373-633X


Case Report Volume 14 Issue 1
1Department of Gynecological Tumors, High Specialty Medical Unit, Oncology Hospital, National Medical Center, XXI Century, Mexican Social Security Institute, IMSS, Mexico
2Oncologic Surgery, High Specialty Medical Unit, Oncology Hospital, National Medical Center XXI Century, Mexican Social Security Institute, IMSS, Mexico
Correspondence: Pliego Ochoa Adrian Dersu, Oncologic Surgery, High Specialty Medical Unit, Oncology Hospital, National Medical Center XXI Century, Mexican Social Security Institute, IMSS, Mexico city, Mexico, Tel +52 9991290385
Received: January 11, 2023 | Published: January 19, 2023
Citation: Hector MG, Dersu POA, Ivett DCC, et al. Limbic encephalitis anti-NMDA receptor (anti-NMDAR) associated with mature cystic teratoma: case report and literature review. J Cancer Prev Curr Res. 2023;14(1):1-3. DOI: 10.15406/jcpcr.2023.14.00509
Paraneoplastic neurological syndromes are a heterogeneous group of conditions caused by immunological mechanisms and not by metastatic disease, metabolic deficiencies, infections, coagulopathy, or adverse effects of cancer treatment. These syndromes can affect any part of the nervous system, damaging multiple or specific areas. Although it is one of the most common paraneoplastic neurological syndromes; limbic encephalitis remains a rare diagnosis; Associated with a wide variety of types of cancer, ovarian teratoma being the most common in women. Autoantibodies directed against tumor antigens explain the mechanism by which the nervous system is affected.1
Germ cell neoplasms represent approximately 20-25% of ovarian tumors, teratomas being the most common type, and may contain tissue from all three embryonic layers (ectoderm, medoserm, and endoderm). Most of these tumors are diagnosed before 50 years of age. They are classified into four categories: mature, immature, malignant, and monoderm. Almost all are cystic and in 10-15% of cases they present bilaterally.2
Limbic encephalitis has been associated with a varying amount of protein production and antibodies against the N-methyl-D-aspartate receptor (NMDAR). Anti-NMDAR encephalitis has recently been described as part of the different types of neurological syndromes that affect the limbic system.2
NMDA receptors are part of the 3 glutamate receptor subtypes. Their location is exclusively neuronal and they are responsible for mediating the reaction generated by the polysynaptic discharge of nociceptive primary afferent fibers. NMDA receptors are associated with the processes of learning, memory, development and neural plasticity, as well as in the process of acute and chronic pain. They intervene in the initiation and maintenance of central sensitization, associated with damage or inflammation of peripheral tissues.3,4
A 23-year-old female, with no significant pathological history; It starts acutely with asthenia, adynamia, indifference to the environment, bradypsychia, dysarthria, emotional lability, verbal aggression, insomnia, and delusional ideas of harm, persecution, and auditory hallucinations. The study protocol began with simple computed axial tomography (CT) of the skull, with no evidence of ischemic or hemorrhagic lesions or space-occupying lesions, other tissues without alterations.
It was decided to perform lumbar puncture with analysis of cerebrospinal fluid (CSF) with a transparent, colorless appearance, leukocytes: 6×10-6/L, mononuclear cells: 66.7%, polymorphonuclear cells: 33.3%, microorganisms were not found, India ink negative, glucose: 74 mg/dl, chlorine: 130.2 mmol/L and lactate: 16 mmol/L.
Four days later, magnetic resonance imaging (MRI) of the skull was performed, reporting brain parenchyma of both hemispheres, basal ganglia, thalamus, stem structure, and cerebellum of normal morphology and intensity (Figures 1 and 2). Likewise, an electroencephalogram (EEG) is performed during wakefulness and sleep, reporting within normal limits and observing epileptiform activity.
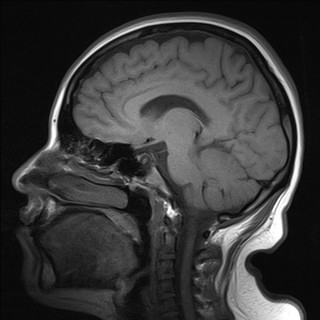
Figure 2 ThreeBrain MRI in sagittal section without evidence of intraparenchymal or structural lesions.
The protocol is complemented with an extension study using an abdominal-pelvic CT scan, documenting a heterogeneous tumor dependent on the right annex with multiple calcifications inside, suggestive of teratoma (Figures 3 and 4).
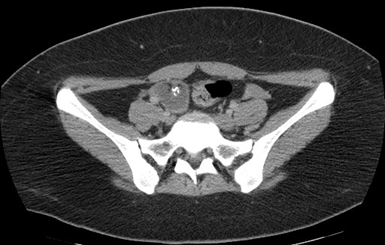
Figure 3 Abdominal and pelvic CT in axial section showing a heterogeneous tumor dependent on the right ovary, round with defined edges with calcium density (393 HU) inside.
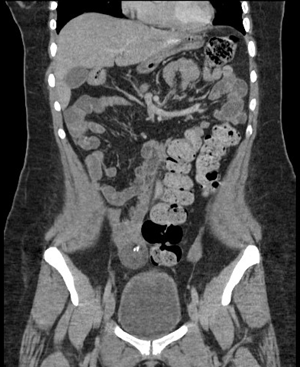
Figure 4 Abdominal and pelvic CT in coronal section showing a heterogeneous tumor dependent on the right ovary with a hyperdense calcium image (393 HU) inside, with peripheral enhancement to the contrast and with dimensions of 3.9 x 3.6 x 4.3 cm and a volume of 30cc.
Complete blood count, blood chemistry, serum electrolytes, thyroid function tests, inflammatory markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were within normal parameters. In addition to negative antinuclear antibodies and normal concentrations of tumor markers including: β fraction of human chorionic gonadotropin hormone (β-HGC), Ca-125 and Alpha-fetoprotein (AFP).
Empirical medical management with intravenous steroid (Methylprednisolone) + Ceftriaxone and Acyclovir was started. A diagnosis of autoimmune encephalitis was established without improvement in the clinical picture despite medical management, so surgical management was decided for the adnexal tumor by means of exploratory laparotomy with right salpingo-oophorectomy, reporting as a finding: right adnexal solid tumor with well-defined borders of 6 x 4 cm, pearly with intact capsule, uterus measuring 7 x 5 cm and left appendage measuring 3 x 2 cm, macroscopically normal. A transoperative study was carried out with a histopathological report of a mature cystic teratoma with a 4.7 cm long axis (Figures 5 and 6).

Figure 5 40x photomicrograph. Section stained with Hematoxylin and Eosin; Alveolar structures with simple squamous epithelium and bronchi covered by ciliated columnar epithelium are observed in the context of a Mature Teratoma.
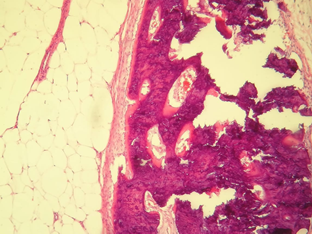
Figure 6 20x photomicrograph. Section stained with Hematoxylin and Eosin; Adipose tissue, fibrous pseudocapsule and osteoid tissue are observed, with bone trabeculae and osteoblasts in the context of a Mature Teratoma.
Antibodies are requested anti-NMDAR, which are reported positive. Treatment with human immunoglobulin for limbic encephalitis is started anti-NMDAR. The patient was discharged three weeks after the onset of symptoms with complete remission of the clinical picture.
The clinical presentation of our patient is consistent with what is described in the literature on paraneoplastic limbic encephalitis; a disorder characterized by a wide variety of neuropsychiatric symptoms: personality changes, irritability, depression, speech disturbances, seizures, amnesia, abnormal movements, and dementia. Some patients also develop signs of diencephalic-hypothalamic dysfunction, including drowsiness, hyperthermia, hyperphagia, and less frequently, pituitary hormone deficiencies.5-10
In the management of limbic encephalitis, it is essential to identify the clinical picture and start targeted treatment as soon as possible. The VITAMINS mnemonic (vascular, infectious, toxic-metabolic, autoimmune, metastatic-neoplastic, iatrogenic, neurodegenerative and systemic) is useful. to systematically consider possible etiologies in these patients.11-15
In 60% of cases, limbic encephalitis is a paraneoplastic disorder. Although the pathogenesis is not fully understood, a humoral cytotoxic T-cell response against neuronal protein antigens (usually expressed by the underlying tumor) is presumed to cause the neurologic symptoms. Depending on the type of cancer, there may be antibodies against surface antigens, for example: antibodies against voltage-gated potassium channels (Anti-CKVD) and Anti-NMDAR, or against intracellular antigens, for example: Anti-Hu (ANNA 1), Anti-Ma2, Anti-CV2 and Anti-Amphifisin.11-15
However, 10 of the 12 patients required orotracheal intubation due to the presence of respiratory distress, requiring ICU stay for an average of 22.5 days. After the surgical intervention, systemic management with steroids, immunoglobulin, plasmapheresis and even Rituximab was continued. Patients were followed for an average of 33 months, with only one patient recovering their initial cognitive function and the majority continuing to experience residual neurological impairment. One patient died of toxic megacolon 83 days after surgery.9 Patients were followed for an average of 33 months, with only one patient recovering their initial cognitive function and the majority continuing to experience residual neurological impairment. One patient died of toxic megacolon 83 days after surgery.9 Patients were followed for an average of 33 months, with only one patient recovering their initial cognitive function and the majority continuing to experience residual neurological impairment. One patient died of toxic megacolon 83 days after surgery.16,17
The limbic system is well known for its involvement in emotions, behavior, motivation, and memory. This disease is characterized by the involvement of said system and can serve as an example for the development of a neuroendocrine model of this entity that up to now has not been well described. The limitations for its development are the number of cases described and long-term follow-up to determine the prognosis of the disease.
The diagnosis of limbic encephalitis as a paraneoplastic syndrome represents a diagnostic challenge, since the most frequent causes such as metabolic and infectious disorders must be ruled out. Imaging studies including CT, MRI, EEG, and lumbar puncture should be performed. When the main causes are excluded, one should inquire about inflammatory/autoimmune disorders.
The Anti-NMDAR encephalitis, it is an under diagnosed disorder, almost always classified as a primary psychiatric condition, a circumstance that delays diagnosis and treatment. Most patients undergo prolonged hospitalization, stay in intensive care units secondary to support with assisted mechanical ventilation, and nosocomial infections. In order to obtain the best therapeutic response, it is necessary to have a high degree of suspicion of this disease.
In young women with a prominent psychiatric condition and after having ruled out the most common causes of encephalitis, the therapeutic approach should include a screening study for adnexal tumors. In patients with ovarian teratoma, a high degree of suspicion of paraneoplastic activity should be held, especially in those with neuropsychiatric symptoms.
The Anti-NMDAR encephalitis, it affects any age group. The presence of an adnexal tumor, generally an ovarian teratoma, is associated with a better prognosis after its resection compared with the use of immunotherapy (corticosteroids and/or intravenous immunoglobulin) than in those patients who are not associated with tumors.
None.
Authors declare that there is no conflict of interest.

©2023 Hector, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.