Journal of
eISSN: 2373-633X


Clinical Paper Volume 14 Issue 2
1Radiation Oncology Unit, IRCCS-CROB, Italy
2Physic Unit, S. Giuseppe Moscati Hospital, Italy
3Physic Unit, IRCCS-CROB, Italy
Correspondence: Grazia Lazzari, Radiation Oncology Unit, IRCCS-CROB, Via Padre Pio 1, 85028, Rionero in Vulture (PZ), Italy, Tel +39 0972 729740
Received: March 19, 2023 | Published: March 30, 2023
Citation: Lazzari G, Becci D, Mola D, et al. Holo-cranial brain sparing total scalp tomotherapy and neoprene cap in diffuse scalp non Hodgkin B lymphoma: does it work? J Cancer Prev Curr Res. 2023;14(2):46-50. DOI: 10.15406/jcpcr.2023.14.00517
Background: Total scalp irradiation could be suggested to treat scalp lesions like in Non Hodgking Lymphoma. However this treatment is a big concern due to the complex shape of the cranial vault . Helical Tomotherapy (HT) has been shown to a versatile and effective technique for the treatment of several scalp malignancies requiring total scalp irradiation, but several effective arrangements are required.
Methods: a 70 year old woman with extensive Primary Cutaneus Diffuse Non Hodgking B Cell Lymphoma (PC-DLBCL) of the scalp, after a partial remission with 4 R-CHOP cycles, needed a consolidation radiotherapy. A treatment with holo-cranial-brain-sparing total scalp HT in simultaneous boost (SIB) technique and a neoprene cap bolus was supposed. The treatment plan was firstly simulated on an anthropomorphic phantom (CIRS- ATOM®) with (plan A cap on ) and without (plan B cap off ) a neoprene suit cap 5 mm thick as bolus.
Results: In plan A with bolus , the 99.6% of PTV 40 received the 95% of the prescribed dose (PD Gy); the D100 (the 100% of PD) was delivered to the 53.7% of volume; no 107% of the PD were recorded. Plan A was chosen to treat the patient. This plan was verified with Mosfet dosimeters on the phantom head and compared with the Gafchromic films EBT3b (Asland®). No difference were found in dosimetry. Treatment planning was carried out on a Tomotherapy Precision planning workstation (Accuray v. 2.0.1.1®). No acute side effects were recorded. The patient reached a clinical and radiological complete remission.
Conclusions: Holo-cranial brain sparing plan in HT and SIB modality with a neoprene suit cap seems an easy, safe and time effective combination to treat diffuse scalp lesions.
Keywords: Holo-brain sparing, scalp, tomotherapy, bolus, neoprene
Primary Cutaneus Diffuse Non Hodgking B Cell Lymphoma (PC-DLBCL) represents 10% of all Non Hodgking Lymphomas involving the trunk, the neck and less frequently the scalp.1 As therapy, after chemotherapy, total scalp irradiation could be suggested although this approach is a big challenge due to several issues like the complex shape of the cranial vault, and the underlying brain, which is critical mainly in elderly patients.2 There is a growing body of evidence indicating HT as a well-suited technique for total scalp irradiation, because of its ability to treat the scalp with tangential beamlets so to achieve more uniform dose to the scalp, lowering the dose to the brain in the context of high-dose regions.3 Further, to ensure an adequate skin dose coverage adding an uniform thickness a bolus could be helpful.4 We present a case of PC-DLBCL with several lesions spread in the scalp successfully treated with HT and a neoprene cap as bolus.
Clinical presentation
Written informed consent was obtained by the patient. A 70 year old woman was referred to our department for a Primary Cutaneus Diffuse Non Hodgking B Cell Lymphoma (PC-DLBCL),1 clinically presenting several nodular masses spread on the scalp and recorded on CT images (Figure 1A, 1B).
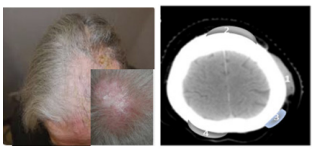
Figure 1 (A) PC-DLBCL nodular mass on the scalp of the patient. (B) The CT scan image of the scalp lesions.
After 4 R-CHOP cycles, four lesions remained so consolidation radiotherapy was prescribed. Total scalp irradiation to the entire scalp and residual lesions were planned with HT (Accuray Radixact X v.9®) and intensity modulated radiation therapy with simultaneous boost (IMRT-SIB) modality in 20 fractions (frs) to treat both targets with different radiation level doses. Prescription doses were 36 Gy to the entire scalp and 40 Gy to the four lesions. Treatment required several dosimetry checks before the delivery to the patient as shown in the flow-chart (Figure 2). Planning target volumes (PTVs) and organ at risks (OARs) were defined (brain, lens, ocular bulbs, optic chiasma, brain stem, hippocampus, pituitary gland). A bolus was supposed to use.
Phantom simulation
Previously, the treatment plan was performed and simulated on an anthropomorphic phantom (CIRS- ATOM®) with (A plan -cap on) and without (B plan -cap off) the neoprene cap. For A plan, a tight cap conformed to the shape of the scalp was applied as bolus. This bolus was a 5 mm thick neoprene suit cap, as present in market. On dose-volume histogram (DVH) evaluation, in the A plan we obtained the best dose distribution. In fact the 99.6% of PTV 40 received the 95% of the prescribed dose (PD Gy); the D100 (the 100% of PD) was delivered to the 53.7% of volume; no 107% hot spots of the PD were recorded. As a result, between the cap on and cap off plans, a better coverage was obtained by the use of the cap bolus, showing a more homogeneity dose and no hot spot doses (Table 1A).
PTV cap -on (A) |
D% Gy |
V % |
PTV 40 |
95 |
99.6 |
" |
100 |
53.7 |
" |
96.5 |
98 |
" |
107 |
3 |
PTV cap- off (B) |
D% Gy |
V% |
PTV40 |
95 |
96.4 |
" |
100 |
56.6 |
" |
96.5 |
98 |
" |
107 |
3 |
Table 1A (A) Difference in dose coverage between plan cap on and cap off. (B) Comparison between the measured and calculated dose in the five points with film and mosfets measurements
Phantom dosimetry checks
Once accepted the plan A, the scalp surface dose was assessed with five Mosfet dosimeters under the cap at the level of the cranial vault, skull base and temporal lobes (Figure 3A). Then dose was verified by with several Gafchromic films EBT3b (Asland®) on the phantom surface (Figure 3B) as suggested by Hardcastle et al.5 By the comparison between the measuared and calculated dose in the five points with both measurements methods, there was a difference of less 5%. This difference is accounted as a normal finding (Table 1B).6
Points |
D Calculated (cGy ) |
MOSFET (cGy) |
Mis vs Calc % |
GAFCHROMIC (cGy) |
Mis vs Calc % |
P1 |
197 |
206 |
4.5 |
188.6 |
-4.3 |
P2 |
175.3 |
184 |
5 |
165.2 |
-5.8 |
P3 |
172.4 |
182 |
5.6 |
155.1 |
-10 |
P4 |
191.2 |
198 |
3.6 |
189.2 |
-1 |
P5 |
177.5 |
175 |
-1.4 |
160 |
-9.9 |
Table 1B
Patient simulation and treatment
Thus the cap-on A plan was chosen to treat the patient. The neoprene cap was worn on the bolt head under a customized thermoplastic mask (Figure 3C). Planning computed tomography images were acquired through the region of interest using a 1 mm slice thickness. Clinical target volume 1 (CTV1) consisted of the entire scalp from the skull base to the frontal skin up to the orbital roof plus a 4 mm margin; then 1 mm was added to obtain the Planning Target Volume 1 (PTV1). The external body included the neoprene external border. Gross tumor volumes (GTVs) were the postchemotherapy lesions. PTVs margins for these GTVs were 3 mm not overpassing the first half of the skull bones. Then margins were cropped 2 mm from the external contour (mask and neoprene cap) to account for dose build up. For PTV1 the prescribed dose was 36 Gy/1.8 Gy. PTVs2 consisted of the 4 residual lesions spread in the scalp defined as GTVs 1- 4 plus margins except on the external border of the cup. The prescribed dose to each PTV2 was 40 Gy/2 Gy. OARs included the whole brain, lens, optic nerves, optic chiasma, pituitary, hippocampus and brain stem (Figure 3D, 3E). Several directional blocking dictates with priorities were given to create a hole to spare brain and eyes as the main OARs (Table 2A, 2B). Treatment planning was carried out on a Tomotherapy Precision planning workstation (Accuray v. 2.0.1.1®).
Name |
Priority |
Max Dose (Gy) |
Max Dose Penality |
DVH Vol (%) |
DVH Dose (Gy) |
Min Dose (Gy) |
Min Dose Penality |
PTV40 P |
100 |
40 |
100 |
65 |
40 |
40 |
90 |
PTV36_P |
100 |
36 |
200 |
95 |
36 |
36 |
300 |
Table 2A (A) Priority dictates for PTVs, (B) Dictates dose references for OAR’s
Name |
Overlap Priority 1 |
Beam Intersection |
Importance |
Max Dose (Gy) |
Max Dose Penality |
DVH Vol (%) |
DVH Dose (Gy) |
DVH Penality |
Len R |
1 |
Never |
5 |
5 |
10 |
50 |
3 |
5 |
Len L |
2 |
Never |
5 |
5 |
10 |
50 |
3 |
5 |
Ocular bulb R |
3 |
Never |
1 |
5 |
1 |
1 |
1 |
1 |
Ocular bulb L |
4 |
Never |
1 |
5 |
1 |
1 |
1 |
1 |
Pituitary gl |
7 |
Allowed |
5 |
// |
// |
// |
// |
// |
Chiasma |
8 |
Allowed |
// |
// |
// |
// |
// |
// |
Brainstem |
9 |
Exit Only |
1 |
40 |
1 |
1 |
1 |
1 |
Hippoc L |
13 |
Never |
1 |
40 |
1 |
1 |
1 |
1 |
Hippoc R |
14 |
Never |
1 |
40 |
1 |
1 |
1 |
1 |
Brain |
20 |
Allowed |
5 |
38 |
10 |
5 |
25 |
5 |
Larynx |
21 |
Exit Only |
2 |
4 |
5 |
10 |
2.5 |
5 |
Oral Cavity |
22 |
Exit Only |
2 |
7 |
5 |
10 |
6 |
5 |
Table 2B
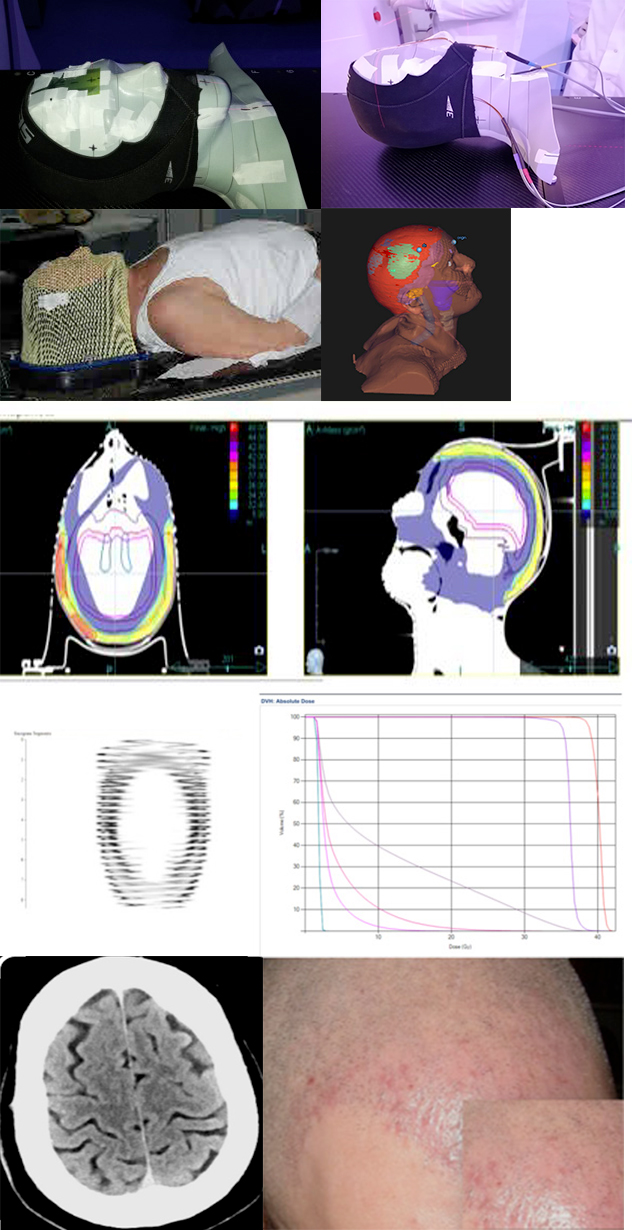
Figure 3 (A) Phantom dosimetry with cap on and gafchromic films. (B) Phantom dosimetry with cap on and mosfet dosimeters. (C) Patient simulation with the neoprene cap under the customized thermoplastic mask. (D) Main frame of plan : PTV 36 ( red cap) and 1 PTV 40 ( green lesions). (E) Plan evaluation with doses distribution. (F) Sinogram of the dose fluence and DVH. (G) Clinical outcome showing a complete remission on the patient (H) CT scan image showing no nodules on the scalp.
To each PTV40 the D98=95.3%, D2=103.4%, D mean 40.1 Gy were recorded. To PTV36 the D98=96.5%, a Dmean = 36.7 Gy were calculated. The Conformity Index (CI) 1.01 and Homogeneity Index (HI) 1 to PTV 40 and CI 1.05 and HI 1.05 to PTV 36 were found respectively. On DVH evaluation lower doses to the brain, lens and chiasma , brain stem were obtained (Figure 3F). The pitch was 0.315, the MF was 2.3 and the jaw size was 2.5 cm. The delivery time was 472 seconds. Daily set up megavolt CT scans (MVCT ) were acquired. A mean set up errors less than 2 mm were recorded. The patient completed the entire treatment without acute symptoms except a diffuse erythema G1 and desquamation of the scalp surface G1 according CTCAE v.5 (Figure 3G). The CT scan after 6 months showed a complete resolution of the disease (Figure 3H).
PC-DLBCL with a low expression of B-CL2 according the update WHO-EORTC 2018 classification is an uncommon type of Non Hodgkin Lymphoma characterized by the skin involvement without signs of systemic disease at initial presentation.1 External beam radiotherapy is effective but it seems a big concern in obtaining a dose uniformity through this target because of the complex anatomy of this site. To solve these critical issues, several attempts have been made to obtain the best dosimetry.
First of all, due to their properties for external surfaced targets, megavoltage electron beams have been applied with different approaches. For example, Mellenberg et al. used several matching electrons fields with gap shifts during treatment.7 To obtain a more uniform dose by eliminating the gap of abutted fields and by increasing the field shift, a technique using six stationary fields was reported by Able et al.8
Moreover to ensure a better homogeneous dose transition between adjacent fields, a more complex technique with combined tangential and normal overlapping electron fields was applied by Walker et al.9
However all these techniques have been found laborious and time-consuming, showing pitfalls in dosimetry. Finally, the quality of the plans have been improved with IMRT due to its ability to homogeneously cover irregularly shaped target volume like the scalp. To this regard, Ostheimer C et al first described an IMRT technique of coplanar and non-coplanar step-and-shoot total scalp irradiation in several cases of lymphoma, obtaining a good dose coverage of the target and low dose to OARs like 4-8 Gy to the lens.10 Moreover, arch therapy as the multijaw-size concave arc technique (MCAT) has been designed using a dynamic conformal arc for the total scalp, with a multileaf collimator to shield the brain. Then two additional conformal arcs with a decreased upper-jaw position of the first dynamic conformal arc to reduce the cranial hot-spots have been added. But MCAT has been shown to be inferior to IMRT with respect to dose homogeneity and over-dosage.11
Another techniques like a double archs VMAT plans have been investigeted in few reports with several arrangements,12 showing an improved dosimetry. Indeed in a comparison with IMRT arrangements, target coverage, homogeneity and OAR protection, have been found slightly superior in VMAT plans,13 showing similar quality with that of 9-field IMRT, but spending a reduced delivery time.14 Attempts with protons are still going on.15
However among IMRT arch techniques, HT has achieved the best dosimetry quality to treat homogeneusly the scalp resulting in a complete resolution in reirradiation (10) or aggressive scenarios as angiosarcoma or progressive scalp T cell lymphoma.16,17
Indeed this technique seems to offer many advantages in comparison with other IMRT linac based plans. By a comparison study of Orton et al. between HT and Linac based plans, the target equivalent uniform dose (EUD) for the best tomotherapy plan was slightly higher than for the Linac plan, while the volume of brain tissue receiving over 30 Gy was reduced by two thirds.18
By another study of Song et al.,19 comparing three different treatment modalities for total scalp irradiation, including the conventional lateral photon–electron technique, helical tomotherapy, and volumetric-modulated arc therapy, the HT plan showed the best target coverage and conformity, with low doses to the brain and hippocampus. Due to the HT ability of tangential beam delivery, resulting in highly conformal and homogenous dose distribution across large, complex target volumes with substantial OAR sparing, it could be pointed out that HT is ideally suited for holo-cranial brain-sparing modality. As shown by Gupta et al, holo-cranial brain sparing techique on several scalp tumors including scalp lymphomas seems feasible to obtain the best dosimetry for scalp and organs at risk with a hole sparing dose to the brain.20
On the basis of this background, we tried to offer this technique to this older patient with the novelty of a SIB and a customized bolus. To this regard, the use of a bolus is still under consideration.
It is well aknowledged that in IMRT, the treatment planning system increases the fluence of tangential beam near the skin surface to counter the build-up region with a consequent increase of dose to the skin surface.21 Thus, bolus is useful in replacing the electron density of the surrounding air. In the meanwhile it is able to suppress the extremely high fluence near the skin eliminating the hot spots dose as clearly demonstrated by Takenaka et al. in a study analyzing the effect of a virtual bolus.22 On the contrary, in the study of Song, the use of bolus seems to not reach any difference in tomotherapy plans.19
Customize a bolus anatomically conformal to the scalp is very difficult owing to the convex shape of the scalp. Several attempts to customize a bolus cap have been made like an Aquaplast® mesh adherent to the patient’s scalp,4 a double shell bolus hand-made12 or a 3D customized printed bolus.23 But all these techniques have resulted in time spending and air keeping under the bolus, producing uncertaines in dosimetry. Further which bolus thickness has been not defined. Lobb et al. suggested that a 5 mm thickness bolus should work well. They showed that a radial expansion of the scalp CTV into 5 mm of bolus material minimizes dosimetric sensitivity to errors in patient position as large as 5 mm.24 In our experience the use of neoprene cap 5 mm thick was safe and effective.
Thus we choose a neoprene 5 mm thick suit hood present in the market, conformal to the head size of the patient as a feasible and time effective tool for our tomotherapy plan. The simulation step on phantom confirmed its dosimetric efficacy according to gafchromic and Mosfet dosimetry data. The of holobrain sparing plan in SIB modality and neoprene cap seem an easy, safe and time effective combination to treat scalp lymhoma.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.

©2023 Lazzari, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.