Journal of
eISSN: 2373-633X


Research Article Volume 13 Issue 3
1Department of Medical Oncology, Faculty of Medicine, Kyorin University, Japan
2Shirakaba Clinic, Japan
3Department of Medical Technology, Faculty of Health Sciences, Kyorin University, Japan
4Department of Medical Technology, Faculty of Health Sciences, Gunma Paz University, Japan
5Department of Infectious Diseases. Faculty of Medicine, Kyorin University, Japan
6Department of Public Health, Faculty of Health Sciences, Kyorin University, Japan
Correspondence: Hiroshi Kitamura, Department of Medical Oncology, Faculty of Medicine, Kyorin University, 6-20-2, Shinkawa, Mitaka-shi, Tokyo, 181-8611, Japan, Tel +81-3-3995-7700, Fax +81-3-3995-7702
Received: June 10, 2022 | Published: June 24, 2022
Citation: Kitamura H, Itoda I, Okodo M, et al. High-resolution anoscopy for the diagnosis and treatment of human papillomavirus-related anal intraepithelial neoplasia in human immunodeficiency virus-seropositive men who have sex with men in Japan. J Cancer Prev Curr Res. 2022;13(3):83-88. DOI: 10.15406/jcpcr.2022.13.00493
Background: In the US and Europe, human immunodeficiency virus (HIV)-seropositive patients who do not have acquired immunodeficiency syndrome (AIDS) and have human papillomavirus (HPV) infection in the anal canal have a significantly increased risk of developing anal cancer. Examination and ablation using high-resolution anoscope (HRA) are widely performed for the treatment of high-grade anal intraepithelial neoplasm (HGAIN), a precancerous condition. The increasing risk of anal cancer by HPV is a concern also in Japan and other Asian countries, but systematic examination and treatment is lacking. Therefore, we performed the first prospective single-arm study of tissue biopsy and HRA treatment in Japan.
Methods: From April 2016 to March 2020 at Kyorin University Hospital and cooperating institutions, we performed anal Pap smears on 125 HIV-seropositive men who have sex with men (MSM) and conducted HRA and tissue biopsy. Additionally, in the case of HGAIN, we conducted ablation.
Results: Among the tissue samples, 43.2% were positive for atypical cells. Fifty-two patients gave consent and underwent HRA and tissue biopsy; low-grade (LG)AIN was identified in 14 patients and HGAIN was identified in 38 patients. Seven patients in the HGAIN group did not consent to abrasion. A total of 105 cumulative HRAs were analyzed for association with independent variables (degree of AIN, high-risk HPV genotype, and patient data). There were significant associations between worse AIN and both the presence of genotype 16 or 68 and the number of receptive anal intercourse without condoms. No cancer was detected in this study.
Conclusion: Together with the risk factors, HRA is useful for the early detection of high-grade precancerous lesions.
Keywords: HPV, HIV, HGAIN, HRA, anal canal cancer
In the United States and Europe, local infection with human papillomavirus (HPV) in human immunodeficiency virus (HIV)-seropositive patients without acquired immunodeficiency syndrome (AIDS) significantly increases the risk of developing squamous cell carcinoma of the anal canal (SCC-AC).1-4
In Japan, data on the frequency of SCC-AC among HIV-seropositive individuals are limited to case reports, and the true percentage is unknown. On the other hand, regarding screening by anal pap smear (APS) cytology, 75.9% of men who have sex with men (MSM) and approximately 20% of heterosexuals in Japan are reported to be infected with HPV, which carries a high risk of carcinogenesis. With the expected increase in HPV-related anal cancer in the future, an algorithm for early diagnosis and treatment is required in Japan. Therefore, we performed APS cytology in HIV-seropositive patients, followed by HRA in the case of positivity and infrared coagulation in advanced anal intraepithelial neoplasia (AIN). AIN is classified into three levels according to depth, with AIN2 and AIN3 considered High Grade (HG) AIN or high-grade squamous intraepithelial lesion (HSIL). We aim to use AIN to describe the depth in detail.
First, we performed HRA on Japanese HIV-seropositive patients based on the results of APS to reveal the prevalence of HGAIN. Next, we examined the efficacy and safety of HGAIN treatment intervention using Infra-red Coagulator (IRC) with HRA. Finally, we examined the association between AIN, patient data, and patient background and high-risk HPV genotypes.
Cytology and classification
Like cervical Pap smears, anal pap smears are classified according to the Bethesda system. APS is divided into negative intraepithelial lesions or malignancy (NILM) and atypical squamous cells (ASC), and ASC is further divided into ASC of indeterminate significance (ASC-US) and ASC of high-grade squamous lesions (ASC-H).5 More severe ASCs are defined as squamous intraepithelial lesions (SILs) and are classified as low-grade SIL (LSIL) and high-grade SIL (HSIL). These findings suggest the presence of precancerous lesions and are considered positive by APS screening if they are ASC-US or higher. Although AIN is sometimes referred to as SIL, to clearly distinguish it from cytology, we intentionally refer to SIL here as the result of cytology and AIN as the result of histology.
AIN originates from the anal transformation zone of the anal canal, like the cervix, and is classified as AIN 1–3 according to depth. Like cervical intraepithelial neoplasia (CIN), histological findings are graded as follows: AIN1, extremely mild to mild dysplasia; AIN2, moderate dysplasia; and AIN3, severe dysplasia and carcinoma in situ. High-grade AIN (AIN2 and 3, HGAIN) refers to the precancerous state, which is associated with persistent infection and high-risk HPV. HGAIN has been highlighted as a cause of anal canal cancer and plays a key role in its development.6,7
Treatment
Infrared coagulation (IRC) under HRA is common treatment for HGAIN, including intraepithelial carcinoma, in Europe and the United States. This method is more curative than cryotherapy or topical agents, less invasive than electrocautery, and can provide larger area ablation.8
Patients and intervention
In this prospective single-arm clinical trial, we performed APS examinations in HIV-seropositive patients receiving treatment at Kyorin University Hospital and Shirakaba Clinic. All patients were provided with written explanations and consent was required prior to participation. The study was conducted in compliance with the Declaration of Helsinki. The ethical committee of the Faculty of Medicine at Kyorin University approved this clinical research protocol (approval no. 575). This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (registration number: UMIN000011546). HRA was performed in patients with ASC-US or higher dysplasia based on APS results. Patient entry and examination, treatment, and follow-up were conducted from April 2016 to March 2020. After obtaining written informed consent, we performed a magnified observation of the anal canal using HRA and tissue biopsy of the abnormal areas for histological diagnosis. Subsequently, we diagnosed the degree of AIN and its distribution on the surface of the anal canal, which was followed by IRC at the site of HGAIN.
Procedures
Based on the protocol recommended by Chin-Hong and Palefsky,6 APS was performed using a treatment algorithm.
Because of the limited number of people, space, and time required for HRA, we partially modified this algorithm (Figure 1). The procedure involves placing the patient in the left lateral recumbency position, inserting an anoscope, magnifying the anal canal with a colposcopy, applying 3% acetic acid, observing the nebulous anal TZ (called Aceto-White lesion) surface, margins, and vascular abnormalities, and collecting five to six biopsy samples in all directions around these irregular surfaces to map the degree of AIN.
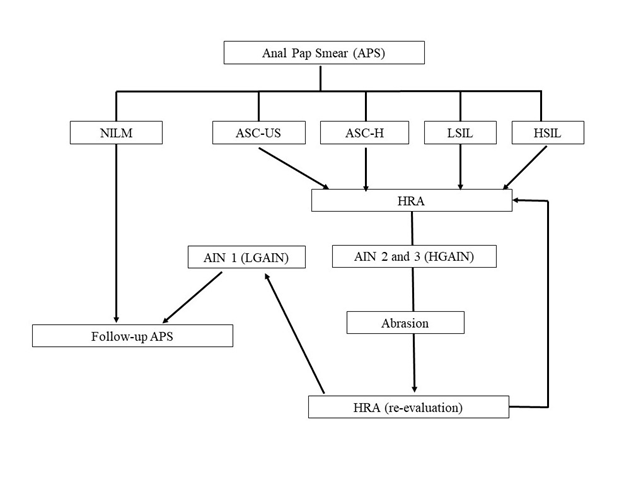
Figure 1 Algorithm for screening, HRA, and treatment.
APS, anal Pap smear; NILM, negative for intraepithelial lesion or malignancy; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells of high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; HRA, high-resolution anoscopy; AIN, anal intraperitoneal neoplasia
When atypical cells showing ASC-US or higher based on the Bethesda system were detected in cytology, HRA was performed, and biopsy was collected from the site of suspected dysplasia. After HGAIN detection, treatment was performed according to the following algorithm: IRC for HGAIN, followed by 3 to 6 months later with HRA and tissue biopsy. Tissue biopsies were performed. This cycle of testing and treatment was repeated until all sites with HGAIN were AIN1. The HRA only and IRC+HRA adverse events were graded using CTCAE v5.0-JCOG (National Cancer Institute, Bethesda, MD).
Primary outcome
The prevalence of Dysplasia (AIN1-3) as revealed by HRA in HIV-seropositive and APS-positive patients.
Secondary outcomes
Secondary outcomes were as follows: 1) HPV genotyping, identify high-risk HPV genotypes corresponding to the occurrence of HGAIN of Japanese HIV-seropositive MSM; 2) AIN worsening factors, relationship between the degree of AIN and patient data (pre-test CD4+ lymphocyte count) and patient background (duration of treatment and no treatment, Brinkman index, number of patients who had passive anal intercourse without condom).
Statistical analysis
We calculated that approximately 45 cases were needed to obtain a significant difference between the APS positive and APS negative groups with a t-test at a significance level of P=0.05. To account for attrition during the study, we set the number of cases at 50 each. Based on our previous study where APS was performed on 81 HIV-seropositive patients, and 40 patients (49.4%) had positive findings of ASC-US or higher, we planned to perform APS on approximately 100 patients in order to obtain 50 patients with positive APS findings. All statistical analyses were performed using EZR ver. 1.54 (jichi.ac.jp).10
APS and the histological diagnosis of biopsy with HRA
In this study, of the 125 patients who underwent APS, 54 patients (43.2%) were positive for ASC-US or higher, an indication of HRA. Fifty-two of the 54 APS-positive patients underwent HRA. Of the remaining two patients, one developed malignant lymphoma and died before HRA and the other refused HRA; when HRA was performed, thirty-eight of the 52 patients (73.1%) had HGAIN, which was an indication of IRC (Table 1).
|
APS (N=125) |
n |
(%) |
Total (%) |
|
Difficult to judge |
35 |
28 |
|
|
NILM |
36 |
28.8 |
|
|
71 (56.8%) |
|||
|
ASC-US |
9 |
7.2 |
|
|
ASC-H |
2 |
1.6 |
|
|
LSIL |
23 |
18.4 |
|
|
HSIL |
20 |
16 |
|
|
54 (43.2%) |
|||
|
Histopathological diagnosis (N=52) |
n |
(%) |
|
|
AIN1 |
14 |
26.9 |
|
|
AIN2 |
16 |
30.8 |
|
|
AIN3 |
22 |
42.3 |
|
|
HGAIN (AIN2+AIN3) |
|
38 (73.1%) |
|
Table 1 APS and the histological diagnosis of biopsy with HRA
Abbreviations: APS, anal pap smear; NILN, negative for intraepithelial lesion or malignancy; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells of high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; AIN, anal intraperitoneal neoplasia; HGAIN, high-grade anal intraperitoneal neoplasia
Sensitivity and specificity of APS for AIN
The sensitivity and specificity of the APS results were examined using a receiver operating characteristic curve with NILM as negative, ASC-US or higher as positive, normal to AIN1 as no disease, and HGAIN (AIN2+3) as the presence of disease. Moreover, the area under the curve (AUC) was 0.720 (p<0.001; Figure 2).
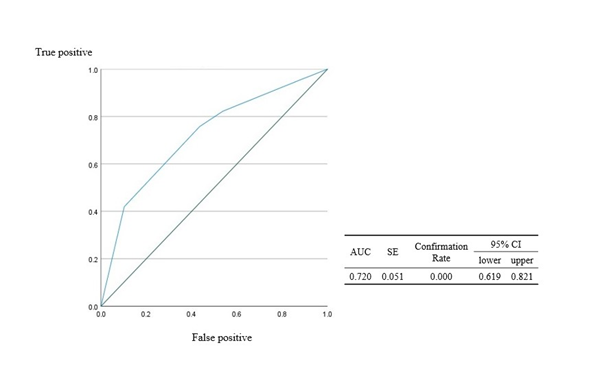
Figure 2 ROC curve and AUC of APS
ROC, receiver operating characteristic; AUC, area under the ROC curve; SE, standard error; APS, anal Pap smear
Patient characteristics and background data
Table 2 summarizes the characteristics and background data of the included participants. The median age was 41 years (range, 24–63 years), and all patients were MSM. We examined CD4+ lymphocyte counts and viral load in the most recent HRA. We also interviewed participants about their current smoking status and calculated the Brinkman index. In addition, we asked about history of condyloma acuminatum and total number of instances of passive anal intercourse without the use of a condom. In addition, the date of HIV seropositivity and the date of antiretroviral therapy (ART) initiation were identified, and the duration of treatment-free and treatment duration were calculated.
|
Patients who underwent HRA (N=52) |
Mean (range) |
|
|
Age |
(years) |
41 (24–63) |
|
Sex and sexuality |
MSM |
|
|
Previous history of condyloma |
(yes:no) |
17:35 |
|
No treatment perioda |
(months) |
7.92 (0.3–264) |
|
Clinical data |
||
|
CD4+ lymphocyte count |
632 (136–1284) |
|
|
Viral load |
Below detection limit |
|
|
Number of receptive anal intercourse sessions without a condom |
10 (1–100) |
|
|
<5 (people) |
13 |
|
|
<10 |
16 |
|
|
<20 |
12 |
|
|
<50 |
6 |
|
|
>100 |
5 |
|
|
Current smoking status |
(yes:no) |
16:36 |
|
Brinkman Index |
n |
|
|
0 |
29 |
|
|
≥1, <400 |
17 |
|
|
≥400, <700 |
4 |
|
|
|
≥700 |
2 |
Table 2 Patient characteristics, background data, and HIV status
aThe date of HIV-seropositive findings and antiretroviral therapy (ART) initiation was confirmed, following which the treatment-free period was calculated.
Abbreviations: HIV, human immunodeficiency virus; HRA, high-grade anal intraperitoneal neoplasia; MSM, men who have sex with men
Prevalence of high-risk HPV genotyping
HPV genotyping was performed on all patients who underwent HRA. The number of overlapping high-risk HPVs tended to increase with the degree of atypia; type 16 tended to be more common in AIN3 (Table 3).
|
AIN |
HPV genotype |
n |
|
AIN1 |
Negative |
5 |
|
(n=14) |
Any one of 16, 52, 56, 58 |
7 |
|
31, 52 or 51, 68 |
2 |
|
|
AIN2 |
Negative |
3 |
|
(n=16) |
One type: 18 or 52 |
3 |
|
Two types: 52, 58 or 58, 68 |
2 |
|
|
Three types: 16, 31, 52 or 33, 52, 56 |
2 |
|
|
Four types; 31, 33, 39, 45 |
1 |
|
|
Five types: 16, 33, 51, 58, 59 or 31, 35, 56, 58, 68 |
2 |
|
|
Six types: 16, 35, 52, 56, 58, 59 or 31, 35, 45, 51, 52, 68 or 33, 39, 51, 56, 59, 68 |
3 |
|
|
AIN3 |
Negative |
2 |
|
(n=22) |
One type: 16, 35, 56, 58, or 68 |
8 |
|
Two types: 16, 59 or 33, 68 |
2 |
|
|
Three types: 16, 33, 68 or 16, 31, 58 or 51, 58, 68 |
3 |
|
|
Four types: 16, 33, 51, 68 or 16, 35, 51, 68 or 16, 52, 56, 58 |
3 |
|
|
Five types: 16, 51, 52, 58, 59 or 16, 31, 33, 51, 52 |
2 |
|
|
Six types: 16, 31, 33, 52, 56, 68 |
1 |
|
|
|
Seven types: 31, 33, 39, 56, 58, 59, 68 |
1 |
Table 3 Prevalence of high-risk HPV genotyping
Abbreviations: AIN, anal intraperitoneal neoplasia; HPV, human papillomavirus
HRA and IRC treatment
Table 4 shows a summary of the HRAs performed. The actual treatment was repeated until the HRA was down staged to AIN1 with repeat testing and biopsy of the treated area. Fourteen of the 52 patients were excluded because of AIN1 at initial diagnosis, 7 of the HGAIN patients refused treatment, and 31 were treated; four dropped out of the study before reaching AIN1; the cumulative number of HRAs was 105 times, the cumulative number of IRCs was 50, and downstaging from HGAIN to AIN1 was 27 cases, with a treatment completion rate of 87% (27/31).
|
Number of patients undergoing HRA (N=52) |
||
|
HRA: one time (N=21) |
n |
|
|
The biopsy revealed AIN1. |
14 |
|
|
The biopsy results were HGAIN, but they did not want treatment. |
7 |
|
|
HRA: two or more times (N=31), cases in which IRC was performed |
||
|
Two observations |
Completed in one coagulation procedure |
16 |
|
Three observations |
Completed in two coagulation procedures |
8 |
|
Four observations |
Completed in three coagulation procedures |
2 |
|
Five observations |
Completed in four coagulation procedures |
1 |
|
Drop out during treatment |
4 |
|
|
Total number of patients who underwent HRA |
105 |
|
|
Total number of patients treated with IRC |
50 |
|
Table 4 Results of HRA and IRC treatment
Abbreviations: HRA, high-resolution anoscopy; IRC, infrared coagulation; AIN, anal intraperitoneal neoplasia; HGAIN, high-grade anal intraperitoneal neoplasia
Adverse events
Table 5 summarizes the adverse events after HRA alone and after IRC; one patient developed a perianal abscess after HRA, although there was no post-examination anal pain or bleeding with HRA. However, this patient had a history of perianal abscesses several times a year, with or without HRA; IRC had more severe adverse events, but they were limited to a few days of pain and bleeding. However, only one case was so painful that the patient had to miss work, but this condition was likely because almost the entire circumferential lesion was cauterized at one time. In addition, all cases of bleeding were associated with obvious causes such as internal hemorrhoids, varicose veins, and easy bleeding.
|
Adverse event |
After HRA (n=105) |
After IRC (n=50) |
|
Anal pain (2-3 days) |
0 |
9 (G1) |
|
Anal hemorrhage (2-3 days) |
0 |
9 (G1) |
|
Perianal abscess |
1 (G2) |
0 |
|
Anal pain (about 1 week) |
0 |
2 (G1) |
|
Anal pain (about 1 month) |
0 |
6 (G1) |
|
Anal pain (over 1 month) |
0 |
2 (G1) |
|
Anal pain (short duration, but enough pain to force the patient to rest from work) |
0 |
1 (G3) |
Table 5 Adverse events after HRA and IRC
Abbreviations: HRA, high resolution anoscopy; IRC, infrared coagulation; G, grade from CTCAE (Common Terminology Criteria for Adverse Events) version 4.0
Relationships between AIN and other factors
The relationship between the degree of AIN, high-risk HPV genotype, patient data, and background characteristics was examined in 105 cumulative HRA. Multiple logistic regression analysis was performed using age, current smoking status, Brinkman index, pre-test CD4+ lymphocyte count, duration of treatment and no treatment, history of condyloma acuminatum, number of instances of receptive anal intercourse without a condom, and number of HRA as independent variables. Genotypes 16 and 68 were significantly associated with worse AIN. In addition, genotype 52 tended to be non-significantly associated with worse AIN (p=0.076). There was also a significant association identified between the number of condomless anal sex and worsening AIN (Table 6).
|
|
Odds ratio |
95% CI |
P-value |
|
|
Lower limit |
Upper limit |
(P<0.05) |
||
|
Number of HRA procedures |
1.40 |
0.449 |
4.35 |
0.0086 |
|
HPV genotype 16 (+) |
6.19 |
2.05 |
18.7 |
0.0013 |
|
HPV genotype 68 (+) |
5.31 |
1.08 |
26.3 |
0.0405 |
|
Number of receptive anal intercourse sessions without a condom |
1.03 |
1.00 |
1.05 |
0.0361 |
Table 6 Adverse events after HRA and IRC
Abbreviations: HRA, high-resolution anoscopy; HPV, human papillomavirus
This is the first prospective interventional study in Japan to investigate the incidence of HGAIN in HIV-seropositive patients who underwent HRA and were treated with positive anal cytology. The relationships among history of condyloma, duration of ART treatment, and number of receptive anal intercourse without condom were examined. Smoking history and status, Brinkmann index, and condyloma history were evaluated because they were associated with APS outcome in the previous study9 but not with AIN exacerbation in the current study.
One limitation of this study is the small number of cases, which is attributed to the low awareness of HGAIN testing and treatment in Japan. Due to many limitations in manpower, location, and time, this study was only able to perform HRA once a month on up to three patients. However, the results showed that 54/125 (43.2%) of the patients were cytologically positive, of which 38/52 (73%) were HGAIN.
HPV genotyping results also revealed that high-risk HPV infection among HIV-seropositive patients is particularly common among MSM in Japan. This is certainly a high prevalence and a target population for therapeutic intervention. This trend has been reported to be similar in other Asian countries, including Japan.4, 11-13 However, there are still no published reports on HRA, tissue diagnosis, HGAIN ablation, or even post-treatment adverse events. Our results are consistent with reports from Europe and the United States, and we hope that our findings will spread awareness that this disease is one that should be addressed with a sense of urgency.
In this study, no association was observed between LGAIN or HGAIN and CD4+ lymphocyte counts. Furthermore, there was no correlation with the previous, immediately preceding CD4+ level. Similarly, no association was observed between AIN and duration of treatment; in response, we calculated the treatment-free period (date of HIV-positive discovery and date of ART initiation) and incorporated it as an endpoint. Nevertheless, no association with the degree of severe dysplasia was observed. These may partly be due to the small number of participants, but more likely to the fact that almost all patients were well adherent, with HIV viral load below the detection limit and CD4+ lymphocyte counts adequately controlled.
Although this study was based on a small number of cases, it was necessary to note the factors with significant associations. First, a higher number of instances of receptive anal intercourse without condom use with different partners was associated with an exacerbation of AIN. This association may be due to the increased risk of exposure to different high-risk HPVs and the increased risk of injury from physical irritation due to friction in the anal canal. The use of condoms and lubricating jelly may be effective in reducing high-risk HPV transmission, mechanical injury, and exacerbation of AIN. Next, HRA and the histological search revealed that most cases with AIN3 contained either HPV type 16 or type 68, which significantly contributed to AIN exacerbation. Type 52 (P=0.076) was also frequently included in AIN3, despite being insignificant. Moreover, its contribution may likely become apparent with more HRA cases. These results suggest that AIN2 cases with HPV types 16 and 68 are at high risk of progressing to AIN3 and that AIN2 is a mid-stage process with greater malignancy. Thus, even when AIN1-2 is detected, it may be possible to predict AIN progression based on the genotype of the HPV detected; there have been many reports on type 16,14-16 while type 68 has received less attention. However, it was suggested that it may correlate with the progression of AIN as well as type 16. Type 68 may be unique to AIN.
For reference, a large HPV detection study in Japan detected 14 oncogenic HPV genotypes (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 67, and 68), with types 58, 16, 52, and 33 being particularly common in MSM4. The number of high-risk HPV genotypes was not associated with AIN exacerbation; however, HPV types with a high risk of carcinogenesis are associated with a high rate of co-infection with other types, similar to CIN. Furthermore, AIN in MSM has a high rate of overlapping infection in the high-risk group.17 In general, type 16 is the most common HPV genotype associated with a malignant phenotype, regardless of the organ of origin. Type 16, together with type 18, is reported to account for about 70% of cervical cancer cases.18 In anal cancer, type 16 is found in 68.5% of invasive cases and 62.9% of HSIL cases.19 In the present study, type 16 was significantly associated with high-risk HPV, as were types 68. However, type 68 was less prevalent, which may be due in part to the lack of invasive cancer cases. Nevertheless, since type 16 showed a significant association, we cannot rule out the possibility of geographic or ethnic differences. Larger HRA studies in Japan are warranted to clarify the histological findings and immunohistological distribution of HPV genotypes in the actual tissues of patients with high-risk HPV as well as AIN or anal cancer.
On the other hand, it is reasonable to expect that the administration of high-risk HPV vaccine would reduce continuous infection and prevent progression to anal cancer given the current knowledge of high-risk genotypes with oncogenic potential. However, the effect of the vaccine on HGAIN remains controversial. Quadrivalent vaccination (HPV-6, 11, 16, 18) of young MSM is reportedly effective in preventing continuous infection with anal HPV and progression to AIN 2 and 3.20 The 9-valent vaccine has also shown promising results in cytology studies from Japan.21 Conversely, another report found that the quadrivalent vaccine was ineffective as a post-treatment adjuvant for HGAIN.22 Nevertheless, it is reasonable to conclude that reducing continuous infection with overlapping high-risk HPVs would be a positive step toward preventing AIN progression,23 and that the development of a therapeutic vaccine that can prevent progression from HGAIN to anal cancer should be a central research target.
In this study, APS was performed on 125 MSM, 43.2% of whom were positive for atypical cells. Positive cases were subjected to tissue biopsy under HRA, 73% of which were HGAIN, and a positive correlation was found between AIN exacerbation and the number of receptive anal intercourse without a condom. HPV 16 and 68 infections were also significantly associated with AIN exacerbations.
The authors thank Mariko Sano, Department of Infectious Diseases, Kyorin University Hospital, for her invaluable contribution to the collection of data on HIV-seropositive patients. She is a certified nurse by the Japanese Society for AIDS Research.
We all have no conflicts of interest.

©2022 Kitamura, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.