Journal of
eISSN: 2373-633X


Research Article Volume 4 Issue 2
1Jinan Baofa Cancer hospital, China
2TaiMei Baofa Cancer hospital, China
3Beijing Baofa Cancer Hospital, China
Correspondence: Baofa Yu, East 50 No, Wangzhuang Industry Park, Changping, Beijing, China, Tel 011-86-10-56152159, Fax 86-13906401276
Received: November 24, 2015 | Published: February 8, 2016
Citation: Jing P, Liu J, Li J, et al. Hapten improved overall survival benefit in late stages of non-small cell lung cancer (NSCLC) by ultra-minimum incision personalized intratumoral chemo immunotherapy (UMIPIC) therapy with and without radiation therapy. J Cancer Prev Curr Res. 2016;4(2):63-71. DOI: 10.15406/jcpcr.2016.04.00116
Aims: To evaluate overall survival time of hapten-enhanced chemo immunotherapy in the treatment of advanced NSCLC by UMIPIC with and without radiation therapy and to analyze the effective of hapten as an immune booster.
Materials and methods: 298 patients with advanced NSCLC were treated with UMIPIC or Intratumoral chemotherapy (ITCT). Some in both groups had also received radiation therapy prior to UMIPIC or ICTC. UMIPIC (86 cases) was delivered intratumorally in combination with a proprietary therapeutic regimens composed of four components including an oxidant, two cytotoxic drugs and a hapten, UMIPIC-R (115 cases) with the same procedures and added common radiation therapy. ITCT was applied the same procedures and regimens only without the hapten, ITCT-R with same procedures as ITCT and added common radiation therapy. All data from four groups were reviewed and analyzed. 201 of NSCLC patients were treated with UMIPIC, 86 of 201 NSCLC had radiation therapy, and 97 of NSCLC patients with ITCT, 48 of 97 NSCLC had radiation therapy. All patients were followed until their death and survival benefits were analyzed. Some biopsies were taken for immunohistochemical staining and observation under electronic microscopy.
Results: Median survival time (OS) was 20 months in UMIPIC (test) and 7 months in ITCT (control) (P<0.001) and 6-months and 1-year year survival rate of the UMIPIC and ITCT were 95.3% vs. 61.6% (P<0.001) and 86 % vs. 32.7% (P<0.001), 2-years survival rate of the UMIPIC and ITCT were 37.2% vs. 6.5% (P<0.01) respectively. For groups with radiation therapy, median survival time (OS) was 16 months in UMIPIC-R (test with radiation therapy) and 7 months in ITCT-R (control with radiation therapy) (P<0.05), 6-months and 1-year year survival rate of the UMIPIC-R and ITCT-R were 97.4% vs. 62.5% (P<0.001) and 76.5% vs. 37.5% (P<0.001), 2-years survival rate of the UMIPIC-R and ITCT-R were 36.5% vs. 18.8% (P<0.05) respectively. Also it was revealed that dendritic cells and CD4+, CD8+ in the immune histochemical staining and observation in EM (Figure 4), further confirmed more activities of immunological response induced by UMIPIC.
Conclusion: Hapten improved clinical survival benefits by UMIPIC with hapten and double cytotoxic drugs conferred a superior survival time for patients with advanced NSCLC compared to ITCT with or without multi fractional radiation therapy. The addition of the hapten in UMIPIC with double drugs demonstrated a significant improvement in terms of prolonged survival time.
Keywordshapten-enhanced immunotherapy, intratumoral chemoimmunotherapy, non small cell lung cancer (NSCLC), ultra-minimum incision therapy
NSCLC, non small cell lung cancer; CRF, case report forms; EROTC, european organization for research and treatment of cancer; RECIST, response evaluation criteria in solid tumors; OS, overall survival; UMIPIC, ultra-minimum incision personalized intratumoral chemo immunotherapy
Lung cancer including non small cell lung cancer (NSCLC) remains the leading cause of cancer-related deaths, accounting for about 221,200 new cases in total cases and 158,040 deaths of all cancer deaths in 2014 in the United States.1-2 Routine clinical treatments include surgery, radiation therapy and chemotherapy. Over 54% of patients with NSCLC at diagnosis were not suitable for operation. The five-year survival rate for all stages combined, however, is only 16%.1 Currently, as a first-line treatment of chemotherapy, several agents clinically approved in targeted therapies for lung cancer have ongoing developments such as bevacizumab (Avastin)3 and erlotinib (Tarceva), as well as the second generation drugs afatinib (BIBW2992).4-5 and crizotinib (Xalkori).6 However, they still exhibit toxicities and limitations due to the differences in molecular and histological profiles of lung cancers.7
In a recent presentation at the ASCO meeting from 30 May to 3 June, a phase III trial of cisplatin and gemcitabine revealed an improvement in overall survival with the addition of necitumumab in patients with squamous NSCLC8 and it shows that combination with immunological antibody could improve the effectiveness of chemotherapy.
Published data of UMIPIC9 describes a new option for lung cancer treatment, as it integrates local chemotherapeutic effect with systematic antitumor immunity by Intratumoral drug delivery only with single cytotoxic drug; it revealed UMIPIC did prolong the survival time of advanced lung cancer patients due to the additive of hapten as an important immune booster. We have applied double cytotoxic drugs in UMIPIC and UMIPIC-R (additional radiation therapy) for treatment of advanced NSCLC with a solution including three components, i.e. an oxidant, cytotoxic drugs (Cytosine Arabinoside: Ara-C, and bleomycin: BLM) and hapten. Previous animal studies showed that clinically approved oxidants can effectively coagulate tumor mass thoroughly by chemical denature or de-bulking of tumor mass as a drug depot for sustaining drug release.9,10 In the last decade, we have tried this treatment using a combination of drugs with or without hapten in 298 late stages of NSCLC patients. The group either received UMIPIC and UMIPIC-R, ITCT and ITCT-R. The data from the two groups were collected and analyzed, and the role of hapten in UMIPIC was further evaluated.
Patient selection and data collection
Patients in advanced stages of NSCLC (totaling 298) were enrolled in the study. Most patients had prior chemotherapy and radiation therapy with filatures. Some were unable to undergo common chemotherapy or radiation therapy due to being elderly and late stage of the cancer. The survival data obtained were analyzed. The test group patients (n=201) received UMIPIC without adjuvant treatments. Eighty-six of them had radiation therapy classified as UMIPIC-R to test whether or not radiation therapy improved the clinical benefit of UMIPIC. Control group patients (n=97) received ITCT. Forty-eight of them had radiation therapy classified as ITCT-R. Patients were informed of the study details and agreed to participate by signing written informed consent. Hospital ethics committee approved this study. Confirmed by imaging and pathologic examination or cytological diagnosis, primary NSCLC patients in advanced stages and/or with metastatic cancers from November 2006 to September 2011 were analyzed. Data was collected from case report forms (CRFs) filed by physicians. There were available contents of clinical characteristics data for UMIPIC, UMIPIC-R and ITCT, ITCT-R (Table1). For each patient, the first follow-up visit was scheduled one month after treatment initiation and then scheduled on a monthly basis. The records were updated after each follow-up visit.
Umipic and itct preparation
The solution of drugs for UMIPIC was freshly prepared before each Intratumoral injection with clinically approved components including an oxidant (H2O2, final concentration 0.01%), cytotoxic drugs (Cytosine Arabinoside: Ara-C, and bleomycin: BLM) and hapten; the solution for ITCT contains an oxidant and cytotoxic drugs, but without hapten.
Treatment delivery of umipic and itct
All patients had a chest CT for lung scan as a pretreatment baseline. Routine examination of cardiopulmonary function was also done. Bucinnazine hydrochloride injection (0.1g) and hemocoagulase atrox for injection (1 KU) were injected intramuscularly with the patient lying supine or laterally for accurate location. Prior to UMIPIC, the patients were asked to fast without water for 14 hours prior to this therapy in order to avoid side effects. After routine disinfection, draping and local anesthesia with 2% lidocaine, the 25 Gauge spinal needle was inserted into the tumor with CT guiding, needle tip in tumor was monitored by CT, the core of the needle was taken out and the inflator was connected and used as a high pressure syringe (inflation device, 30atm/bar, Merit Medical, Utah, USA), then the injection of solution was performed.10
UMIPIC and ITCT have the same therapeutic procedure, which are minimally invasive and simple like a needle biopsy and were delivered by a spinal needle inserted into the tumor. The solution was pressurized (at the level of atmospheric pressure) to obtain full distribution of clinically approved regimens in the tumor under CT imaging guidance. Special attention was needed for monitoring the density changes of CT value at margins of tumor to ensure full distribution of drugs to the margins and related complications such as hemorrhage around the needle track post treatment. The drug solution was distributed in the tumor easily since the drug’s solution is water soluble. It is better than oil-drug emulsion which is sticky and hard to distribute in tumors. With a high pressure, the combination of drugs in UMPIC or ITCT could penetrate to full matrix of tumor, even into tumor cells with sustained drug release. The average time of the whole procedure is about 30-45 minutes. The volume of the injection was calculated by the diameter of tumor (Dt) times 2 for 1cm to 5cm of tumors and Dt times 1.5 for not smaller than 6 cm of tumor; Successful treatment according to this calculation is dependent upon attention to this formula in order to deliver enough dosage into tumors. Second and third cycles of treatment were usually required for better efficacy in some patients until the tumor was stable. The patients should be re-examined by CT four to six weeks after the last therapy. Some patients were treated with more cycles of treatments. Some biopsies were taken during CT examination for immunohistochemical staining and observation under electronic microscopy.
Umipic-r and itct-r preparation
Some patients in groups of UMIPIC-R and ITCT-R had additional multi fractionated radiation therapy following UMIPIC and ITCT, with Siemens linear accelerator, 6 Mev X-ray, total given dose was 60 -65 Gy, 5 days per week.
Assessment
The response to treatment was evaluated by solid tumor effect evaluation criterion of EROTC (European Organization for Research and Treatment of Cancer) and RECIST (Response Evaluation Criteria in Solid Tumors) made by the National Cancer Institute (US) [11]. All case report forms (CRF) were filled by treating physicians of study hospitals.
Statistical Analysis
The statistical analysis was done by experts of the Medical College. The primary objective was to evaluate the overall survival (OS), which was defined as the duration from the first treatment to patient death and was estimated using Kaplan-Meier analysis. Comparison of effective rate was calculated with the Chi-square test. Statistical analysis was conducted with SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA); P value of <0.05 and <0.01 was considered of statistical significant difference. Some immunohistochemical staining and electronic microscopy observation was done by experts for studying tumor inflammation, CD4+, CD8+, and dendritic cells.
Patient characteristics: at the end of follow-up, 298 patients enrolled and completed the study. They were randomized into four groups of UMIPIC, UMIPIC-R, ITCT, and ITCT-R. Overall survival data of each group were analyzed, including 201 patients received UMIPIC and 86 of them had radiation therapy in UMIPIC-R; 98 patients received ITCT and 48 of them had radiation therapy in ITCT-R. In the UMIPIC group, 54 (62.8%) cases were male and 32 (37.2%) were female, aged between 22-78 years old with a median age of 56; 91.9% were diagnosed as in stage III and according to tumor-node metastasis (TNM) classification, histopathology of UMIPIC group indicated that 42 (48.8%) of the patients had squamous cell carcinoma, 26 (30.2%) had adenocarcinoma cell carcinoma, and 18 (20.9%) had uncharacterized lung cancer (based on biopsy diagnosis); in the UMIPIC-R group, 78 (67.8%) cases were male and 37(32.2) were female, aged between 19-79 years old with a median age of 54.8; 92.2% were diagnosed as in stage III. Histopathology of UMIPIC-R group indicated that 53 (46.1%) of the patients had squamous cell carcinoma, 34 (29.6%) had adenocarcinoma cell carcinoma, and 28 (24.3%) had uncharacterized lung cancer (based on biopsy diagnosis). In the ITCT group, 32 (65.3%) were male and 17 (34.7%) were female, aged between 20-81 years old with a median age of 58; 89.8% were diagnosed as in stages III and IV. Histopathology of ITCT group indicated that 23 (46.9 %) patients with squamous carcinoma, 7 (14.3 %) with adenocarcinoma, and 19 (38.8%) with uncharacterized lung cancer (based on biopsy diagnosis); in the ITCT-R group, 37 (77.1%) were male and 11 (22.9%) were female, aged between 21-77 years old with a median age of 60; 91.7% were diagnosed as in stages III and IV. Histopathology of ITCT group indicated that 22 (45.8 %) patients with squamous carcinoma, 7 (14.6%) with adenocarcinoma, and 19 (39.6%) with uncharacterized lung cancer (based on biopsy diagnosis). For tumor size of two groups, 54 cases (62.8%) with more than 5 cm in UMIPIC, 64 (55.75%) with more than 5cm in UMIPIC-R while 24 cases (44%) with more than 5 cm in ITCT and 26 (54.2%) with more than 5cm in ITCT-R. The baseline characteristics of the patients were well balanced between the UMIPIC, UMIPIC-R and ITCT & ITCT-R four groups (Table 1).
|
UMIPIC-R |
ITCT |
ITCT-R |
||||||
|
N |
% |
N |
% |
N |
% |
N |
% |
|
Enrolled patients |
86 |
100 |
115 |
100 |
49 |
100 |
48 |
100 |
|
Sex |
Male |
54 |
62.8 |
78 |
67.8 |
32 |
65.3 |
37 |
77.1 |
Female |
32 |
37.2 |
37 |
32.2 |
17 |
34.7 |
11 |
22.9 |
|
Median age |
56 |
54.8 |
58 |
60 |
|||||
Age range |
22-78 |
19-79 |
20-81 |
21-77 |
|||||
Histology |
Squamous carcinoma |
42 |
48.8 |
53 |
46.1 |
23 |
46.9 |
22 |
45.8 |
Adenocarcinoma |
26 |
30.2 |
34 |
29.6 |
7 |
14.3 |
7 |
14.6 |
|
Large cell carcinoma |
0 |
0.0 |
0 |
0.0 |
0 |
0.0 |
0 |
0.0 |
|
Other |
18 |
20.9 |
28 |
24.3 |
19 |
38.8 |
19 |
39.6 |
|
Stage Of disease |
Stage I |
0 |
0.0 |
0 |
0.0 |
0 |
0.0 |
0 |
0.0 |
Stage II |
7 |
8.1 |
10 |
8.7 |
5 |
10.2 |
4 |
8.3 |
|
Stage III |
35 |
40.7 |
49 |
42.6 |
21 |
42.9 |
21 |
43.8 |
|
Stage IV |
44 |
51.2 |
57 |
49.6 |
23 |
46.9 |
23 |
47.9 |
|
Tumor size |
<2cm |
5 |
5.8 |
8 |
7.0 |
3 |
6.1 |
4 |
8.3 |
2–5cm |
27 |
31.4 |
43 |
37.4 |
22 |
44.9 |
18 |
37.5 |
|
>5cm |
54 |
62.8 |
64 |
55.75 |
24 |
49.0 |
26 |
54.2 |
|
Prior treatment |
Chemotherapy |
20 |
23.3 |
25 |
21.7 |
20 |
40.8 |
16 |
33.3 |
Radiotherapy |
45 |
52.3 |
25 |
21.7 |
11 |
22.4 |
10 |
20.8 |
|
Surgery |
2 |
2.3 |
3 |
2.6 |
2 |
4.1 |
1 |
2.1 |
|
None |
19 |
22.1 |
62 |
53.9 |
16 |
32.7 |
21 |
43.8 |
|
Disease status |
Locally advanced |
42 |
48.8 |
57 |
49.6 |
24 |
49.0 |
25 |
52.1 |
Metastatic disease |
44 |
52.2 |
58 |
50.4 |
25 |
51.0 |
23 |
47.9 |
|
Table 1 Patient clinical characteristics
Survival evaluation
there was a significant difference in the overall survival (OS) time between UMIPIC and ITCT. It showed that the median OS in the UMIPIC was 20 months while OS in the ITCT was only 7 months (P <0.001), respectively; also it showed that the median OS in the UMIPIC-R was 16 months while OS in ITCT-R was 7 months (P<0.05), respectively (Table 2). It represented a significant improvement for survival time in UMIPIC compared to ITCT (13 months longer) (P <0.001), a significant improvement for survival time in UMIPIC-R compared to ITCT-R (9 months longer) (P <0.05); there are no significant difference between UMIPIC and UMIPIC-R, ITCT and ITCT-R. With a statistical significant difference, the 6-month survival rate was 95.3% (UMIPIC) vs. 61.6% (ITCT) (P<0.001), 97.4% (UMIPIC-R) vs. 62.5% (ITCT-R) (P<0.001) and 1-year survival rate of 86% (UMIPIC) vs. 32.7% (ITCT) (P<0.001), 76.5% (UMIPIC-R) vs. 37.5% (ITCT-R) (P<0.001); 2 years survival rate of 37.2% vs. 6.5% (P<0.001), 36.5% (UMIPIC-R) vs.18.8% (ITCT-R) (P<0.05), respectively (Table 3), for these two groups depicted in (Figure 1). There are no significant difference between UMIPIC and UMIPIC-R, ITCT and ITCT-R. Notably, the UMIPIC & UMIPIC-R and ITCT & ITCT-R both with more than two cycles of treatment, some had four to five treatments for more powerful de-bulking in main tumor mass and it revealed a significant improvement in survival rate in UMIPIC compared to ITCT.
Group |
N |
Mean (M) |
Median (M) |
log-rank |
|
χ2 |
P |
||||
1. UMIPIC and UMIPIC-R |
|||||
UMIPIC |
86 |
22.8 |
20 |
0.565 |
0.452 |
UMIPIC-R |
115 |
21.3 |
16 |
||
2. UMIPIC and ITCT |
|||||
UMIPIC |
86 |
22.8 |
20 |
43.585 |
0.000 |
ITCT |
49 |
9.4 |
7 |
||
3. UMIPIC-R and ITCT-R |
|||||
UMIPIC-R |
115 |
22.2 |
16 |
4.672 |
0.031 |
ITCT-R |
48 |
15.2 |
7 |
||
4. ITCT and ITCT-R |
|||||
ITCT |
49 |
9.4 |
7 |
2.942 |
0.086 |
ITCT-R |
48 |
15.2 |
7 |
||
Table 2 Comparison of survival time between UMIPIC and ITCT
Group |
N |
6-month survival rate |
2-year survival rate |
|||||||
% |
χ2 |
P |
% |
χ2 |
P |
% |
χ2 |
P |
||
1. UMIPIC and UMIPIC-R |
||||||||||
UMIPIC |
86 |
95.3 |
0.611 |
0.435 |
86.0 |
2.854 |
0.091 |
37.2 |
0.010 |
0.920 |
UMIPIC-R |
115 |
97.4 |
76.5 |
36.5 |
||||||
2. UMIPIC and ITCT |
||||||||||
UMIPIC |
86 |
95.3 |
21.547 |
0.000 |
86.0 |
40.045 |
0.000 |
37.2 |
15.708 |
0.000 |
ITCT |
49 |
61.6 |
32.7 |
6.5 |
||||||
3. UMIPIC-R and ITCT-R |
||||||||||
UMIPIC-R |
115 |
97.4 |
37.733 |
0.000 |
76.5 |
22.676 |
0.000 |
36.5 |
4.975 |
0.026 |
ITCT-R |
48 |
62.5 |
37.5 |
18.8 |
||||||
4. ITCT and ITCT-R |
||||||||||
ITCT |
49 |
61.6 |
0.017 |
0.897 |
32.7 |
0.250 |
0.617 |
6.5 |
3.567 |
0.059 |
ITCT-R |
48 |
62.5 |
37.5 |
18.8 |
||||||
Table 3 Comparison of survival rate between UMIPIC and ITCT
The effective rates (CR+PR+SD/TOTAL) were not analyzed between UMIPIC and ITCT in this study, because our published data indicated no significant difference between UMIPIC and ITCT.9 It is necessary to note that the slight size increase of the tumor mass was observed clinically in both groups at first CT examination, which was likely due to inflammatory response induced by coagulation. With the addition of hapten in UMIPIC, more activities of immunological response with inflammations were induced.12,13 Encouragingly, some of the remarkable responses in advanced cancer patients provided proof of the greatest effect in UMIPIC (Figure 2). Also it was found that dendritic cells and CD4+, CD8+ in the immunohistochemical staining and observation in EM (Figure 3), further confirmed more activities of immunological response induced by UMIPIC.
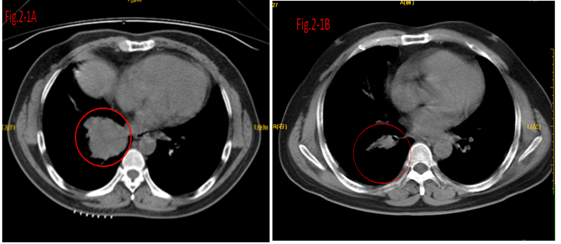
Figure 2Clinical response of UMIPIC therapy in lung cancer.
Figure 2.1 Response of UMIPIC therapy in lung tumor. The patient is a 59-year-old female diagnosed with lung cancer, Adenocarcinoma, sited in the right lobe center. She received a total of 6 UMIPIC injections.
Figure 2.1 (A) The tumor with a diameter of 4 cm pre-treatment and
Figure 2.1 (B) The cardinal of the tumor mass regressed to complete remission (CR) post-treatment (three months).
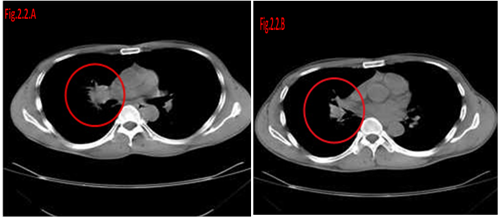
Figure 2.2 Response of UMIPIC therapy in lung tumor. The patient is a 69-year-old male, with inoperable advanced lung cancer of squamous carcinoma at the time of diagnosis. He received a total of six UMIPIC-Therapies.
Figure 2.2(A) Tumor size was 5.4×4.1cm pre-treatment.
Figure 2.2(B) Tumor was regressed to partial remission (PR) post treatment (four months).
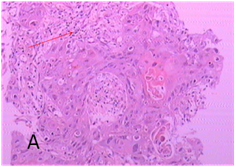
Figure 3 Lung cancer biopsy samples for expression of inflammation invasion, fibrosis under specific staining (EM) and immunohistochemical staining and observation under the electronic microscopy (EM).
Figure 3A In UMIPIC with double drugs, after 7 days of treatment, biopsy was taken to obtain pathological sections for HE staining and showed inflammation invasion with lymphocytes.

Figure 3B In UMIPIC with double drugs, after 7 days of treatment, biopsy was taken to obtain pathological sections for immunohistochemical staining and showed CD4+ lymphocytes activities in tumor cells.

Figure 3C In UMIPIC with double drugs, after 7 days of treatment, biopsy was taken to obtain pathological sections for immunohistochemical staining and showed CD8+ lymphocytes activities in tumor cells.
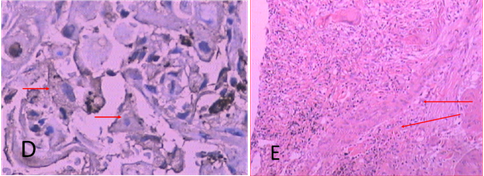
Figure 3D In UMIPIC with double drugs, after 7 days of treatment, biopsy was taken to obtain pathological sections for immunohistochemical staining and showed CD80+ lymphocytes activities in tumor cells, it means dendritic cells were here for tumor antigen presenting.
Figure 3E In UMIPIC with double drugs, after 7 days of treatment, biopsy was taken to obtain pathological sections for specific staining including elastic fiber staining, reticular fiber staining and collagen staining.

Figure 4A-1 After UMIPIC, biopsy was taken for to obtain pathological EM sections and found lung cancer cell with more cell nucleolus and nucleolus ectopia.
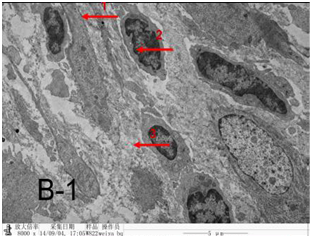
Figure 4B-1 After UMIPIC, biopsy was taken for to obtain pathological EM sections and found that stromal fibroblasts with elastic fiber and collagen within cancer cells.
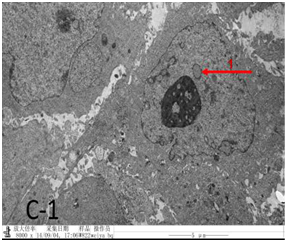
Figure 4C-1 After UMIPIC, biopsy was taken for to obtain pathological EM sections and found that cancer cell was type two pulmonary epithelial cells with arranged in disorder and pseudo-inclusion body.
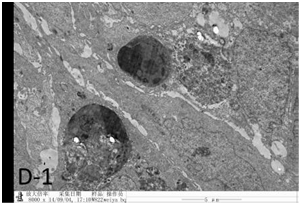
Figure 4D-1 After UMIPIC, biopsy was taken for to obtain pathological EM sections and found that cancer cell apoptosis induced by chemo drugs, epithelial cell linked with cancer cell, tumor cell phagocytosis debris of died cancer cells.
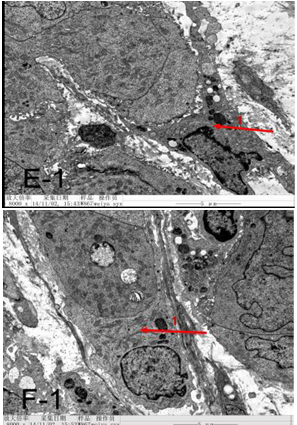
Figure 4E-1 & F-1 After UMIPIC, biopsy was taken for to obtain pathological EM sections and found that dendritic cells with tumor cells.
There was not observed significant differences between the local advanced and metastasis of NSCLC in UMIPIC and UMIPIC-R (Table 4,5).
Group |
N |
Mean (M) |
Median (M) |
log-rank |
|
χ2 |
P |
||||
1. UMIPIC-R-L and UMIPIC-R-M |
|||||
UMIPIC-R-L |
57 |
20.7 |
16 |
0.171 |
0.679 |
UMIPIC-R-M |
58 |
21.8 |
15 |
||
2. UMIPIC- L and UMIPIC- M |
|||||
UMIPIC-L |
42 |
23.9 |
20 |
0.349 |
0.555 |
UMIPIC-M |
44 |
21.8 |
17 |
||
Table 4 Comparison of survival time in local advanced and metastasis of NSCLC between UMIPIC and UMIPIC-R groups
Group |
N |
6-month survival rate |
1-year survival rate |
2-year survival rate |
||||||
% |
χ2 |
P |
% |
χ2 |
P |
% |
χ2 |
P |
||
1. UMIPIC-R-L and UMIPIC-R-M |
||||||||||
UMIPIC-R-L |
57 |
96.5 |
0.360 |
0.548 |
74.1 |
0.003 |
0.958 |
36.8 |
0.005 |
0.944 |
UMIPIC-R-M |
58 |
98.3 |
77.6 |
36.2 |
||||||
2. UMIPIC- L and UMIPIC- M |
||||||||||
UMIPIC-L |
42 |
97.6 |
0.954 |
0.329 |
90.4 |
0.287 |
0.592 |
38.1 |
0.028 |
0.868 |
UMIPIC-M |
44 |
93.2 |
84.1 |
36.4 |
||||||
Table 5 Comparison of survival ratein local advanced and metastasis of NSCLC between UMIPIC and UMIPIC-R groups.
Complication
The related complications include temporary mild fever (not over 380C) for a few hours, minor pain at injection area, and pneumothorax in 8 cases. There were no patients with hemorrhage around the tumor and needle track after therapy. No significant systematic or local adverse effects were observed, and side effects such as myeloid suppression, neutropenia and thrombocytopenia and GI toxicity, apparent loss of hair or appetite were not observed.
NSCLC is still one of the major deadly diseases worldwide. Local treatment like surgery and radiotherapy are the primary curative therapies for patients in early stages of lung cancer. About 54% of patients present a metastasis at diagnosis due to lack of clinical symptoms at early stages, which tend to result in an extremely poor prognosis with an overall 5-year survival rate of 3.8%.3 For most advanced lung cancers, standard chemotherapy generally is the mainstream of management involving Pemetrexed14-15 Oxaliplatin16 and Docetaxel,17 but apparently it has reached a plateau with disappointing outcomes.18 Despite the introduction of a series of targeted drugs for patients with epidermal growth factor receptor (EGFR) mutations (Gefitinib or Erlotinib)19-20 and ALK rearrangement (Crizotinib)20 in the past decade, the survival rate still has not been significantly improved. The use of combination chemotherapy as first line treatment for patients with advanced NSCLC resulted in a statistically better overall survival compared to single agent chemotherapy,21 however, multi-chemo drugs for chemotherapy is reached to consensus for treatment for all solid malignancy tumors.
Today, immunotherapeutic interventions including vaccine therapy derived from lung cancer cell lines (or tumor associated antigens) and immune-stimulatory checkpoint antibodies, may improve outcomes in lung cancer, although traditionally this is not considered to be a possible treatment for tumor. Moreover, the combination of immunotherapy and chemotherapy or chemoimmunotherapy has been successfully applied clinically.22-25 UMIPIC9,10 in this clinical study is a patented therapeutic method for solid tumor, and was explored in this clinic with personalized dosage based on tumor-size while utilizing patient-specific in vivo modified autologous tumor antigens of patient as a self-vaccination to tumor-specific response. The regimen is a personalized and freshly prepared compound solution containing an oxidant, a cytotoxic drug and hapten. Each component plays a vital role in the therapy.
Intratumoral therapy, characterized as high local drug concentrations with minimal systematic toxicity, is an outstanding and attractive alternative to systematic treatment with increasing evidence of its clinical benefits over many years,26-27 but there remains a short time of sustaining the drug effectiveness. UMIPIC, a new intratumoral delivery approach, integrated with the coagulation induced by the oxidant, can significantly increase the local accumulation of drugs for a long time.9,10,28 The coagulation can effectively change the extracellular matrix (EM) and alter the morphological and biochemical components of the tumor such as collagen, elastic fibers, reticular fibers, fibronectin, proteoglycans, hyaluronic acid and other large molecules, obtaining a soft, semisolid, destroyed tumor’s metabolism. It resulted in the sustaining of drugs in the local area of tumor to continue to act in killing of tumor cells and inducing of fibrosis generation to limit the generation of tumor cells (Figure 3). Destroying the environmental conditions for tumor cell growth was proven in our previous animal experiment.29 Therefore, coagulation is one of the major causes of improving drug utilization by extending the duration of drug action, with greatly reduced toxicity.9,30 UMIPIC therapy integrated with surgery, multi drug chemotherapy plus radiation therapy and hapten induced immunotherapy into one new therapy (UMIPIC), has the three functions in one at the same time (Figure 2).
Compared with traditional chemotherapy, the side effects of UMIPIC include mild fever, local pain and accidental pneumothorax in a few cases in this study, but all with an improved quality of life. Pneumothorax is one of the related complications of biopsy in the chest.31 It only happened in a few patients and all recovered spontaneously afterwards. However, to reduce the possibility of pneumothorax, it is suggested to keep the syringe with compounding solution before withdrawing the needle. The patients were asked to inhale to block the needle track, so no cases with local metastasis were found in our study.
Creating an in-situ vaccine depot in the tumor due to tumor-specific antigen is another intriguing factor in the process of intratumoral chemotherapy.32 Furthermore, UMIPIC can not only induce the tumor in-vivo vaccine-like effect, but also enhance systematic immunity due to the addition of hapten.9,10 When multiple autologous tumor antigens were released from the tumor coagulation, cell death can be a priming event for T cell response and can induce potent immunity. These cell deaths were called a “good death”33,34 which elicit a weak immune response as an in vivo self-vaccination promoted by immunologic modulator, i.e. small molecule hapten inlaying the denatured tumor; the modified cell debris or matrixes with tumor antigens became a new complex, more specific to the host immune system. In view of the optimistic survival advantage of UIMPIC with double cytotoxic drugs compared to ITCT, we further analyzed the UMIPIC comparison with published UMIPIC with single drug.10 The median OS of UMIPIC with single drug was 11.23 months but the UMIPIC with double drug was 20 months. One year survival rate 45.45 % of UMIPIC with single drug but UMIPIC with double drugs was 86 %. This indicated the UMIPIC with double drugs for this treatment does sufficiently prolong the survival time and survival rate compared to UMIPIC with single drug. It may contribute to the long term immunological memory and more effective antitumor response from the constitutive releasing of antigens in UMIPIC with double drugs, correlative with dendritic cell and CD4+, CD8+ in immunohistochemical staining (Figure 3). Overall survival time and rate in UMIPIC and ITCT compared to that in UMIPIC-R and ITCT-R, it was not observed significant differences (Table 2 & 3). Therefore it indicated that multi fractionated radiation therapy may not improve the immunological function to elicit cancer cells since daily radiation therapy can damage T cells in the tumor. There was not observed significant differences between the local advanced and metastasis of NSCLC in UMIPIC and UMIPIC-R (Table 4 & 5), it indicated that UMIPIC or UMIPIC-R can prolong OS of NSCLC patients whether in local advanced or metastasis stages.
The systematic immunity against patient-specific tumor associated antigens was significantly boosted by increasing the presentation of antigens induce by chemotherapy or modified with hapten via APCs (including DC and macrophage) to class I and class II pathways (to CD4+ and CD8+ T cells), respectively. It has the potential to generate responses of immune effectors and immune-memory,35-37 to recognize and destroy the residual lung cancer cells that initial coagulation missed in and around the primary tumor and micro-lesions after the UMIPIC. Examples of the elevation of systematic immune response were observed in our animal trial with high levels of CD4+/CD8+28 and some patient biopsy samples.
In summary, UMIPIC with double drugs is a comprehensive procedure and offers new remedies for treating of NSCLC. It not only provides de-bulking or chemical surgery for big tumor mass, but slow release drugs intratumorally continue to kill the residual tumor cells and provide induced systemic immunotherapy to synergistically eradicate the residual tumor cells of whole body in the guardianship to defend against the tumor recurrence and metastasis. This idea seems counterintuitive, even unconventional, and meets the resistance with misunderstanding from varied views of experts in the past years, but now it will likely meet the improvement of freedom in the medical environment since cancer immunotherapy is getting more and more attention every day. It is a potentially effective therapy and on it’s way to reaching a recognized reality with experts such as medical oncologists, radiation oncologists, and surgeons. In fact, it offers a prospect of tailoring treatments much more precisely and could lead to a better survival time, especially in patients in advanced stages of inoperable or drug-resistant types of NSCLC or any solid tumor. Surely, more effective control of the disease and defense of the tumor recurrence or metastasis is needed for us to investigate the UMIPIC with not only double cytotoxic drugs but also double haptens into clinical study, combined with hyper fractionated radiotherapy; it may produce stronger and varied types of immunological responses to eradicate the residual ones of tumors and give more effective options in cancer treatment.
The authors declare no conflict of interest.
None.
None.

©2016 Jing, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.