Journal of
eISSN: 2373-633X


Research Article Volume 2 Issue 2
1AlAhrar General Hospital, Sharkia, Egypt
2University of Benha, Kalyoubia, Egypt
3University of Zagazig, Sharkia, Egypt
Correspondence: Manal Ahmed El Refaei, Ali Zaki tower (15) (8), Zagazig, Sharkia, Egypt, Tel 2.01E+11
Received: January 19, 2015 | Published: March 14, 2015
Citation: El Refaei MA, Refaat MM, El Tohamy MF, et al. Feasibility of different b-values of MRI diffusion in quantitative assessment of liver fibrosis. J Cancer Prev Curr Res. 2015;2(2):36-41. DOI: 10.15406/jcpcr.2015.02.00032
Purpose: We aimed to assess the accuracy of hepatic ADC and the best b-value for diffusion-weighted imaging (DWI) for quantification of liver fibrosis, aiming to improve diagnostic accuracy, in order to achieve a reliable non-invasive marker of hepatic fibrosis.
Materials & methods: One hundred patients with history of chronic hepatitis C virus and twenty five healthy adult volunteers control group were investigated by diffusion MRI in the axial plane, during a single end expiratory breath-hold at different b-values of (200, 500, 700 and 1000s/mm2) on the same day before liver biopsy. ADC maps were generated and the mean hepatic ADC was calculated as the arithmetic mean of the four hepatic ADC values calculated at each b-value. Liver ADCs were correlated with fibrosis scores using the Spearman’s rank correlation coefficient, while Receiver Operating Characteristic (ROC) curve analysis was used to determine the area under the ROC curve (AUC), and the threshold ADC was used to maximize the average of sensitivity and specificity.
Results: The patient group were stratified pathologically according to modified Ishak classification as stage 1 to stage 6 (n=14, 40, 28, 5, 28, 4 and 9 patients respectively). We achieved negative correlation between the ADC values and the degree of liver fibrosis at b-values 200 and 1000s/mm2, respectively, with P=0.000. The ROC curve analysis at b-value =1000s∕mm2, revealed significant difference in ADC values between patients with early fibrosis (F≤4) and those with cirrhotic liver (F≥5) (p=0.000), where the best cut off ADC value to distinguish between these groups was 1.12 x 10-3mm2/s, with 80.5% sensitivity and 77% specificity.
Conclusion: Liver ADC showed a significant difference in the ADC values of non-fibrotic and cirrhotic patients, with high sensitivity and specificity at b-value 1000 s/mm2 with cut off value 1.12 x 10-3mm2/s.
Keywords: liver fibrosis, diffusion weighted MRI, ADC (apparent diffusion coefficient), b-value
DWI, diffusion weighted imaging; ADC, apparent diffusion coefficient; ROI, region of interest; ROC, receiver operating characteristic curve; AUC, area under the curve
Chronic liver diseases encompass many different causes, including mainly viral infections. They constitute an important cause of morbidity, mortality, and health care costs.1 Liver fibrosis is a common feature of almost all causes of chronic liver disease, where it tends to progress, leading to hepatic dysfunction, portal hypertension, and ultimately cirrhosis.2 Liver fibrosis is reversible while liver cirrhosis is not.3 Percutaneous liver biopsy is the gold standard for evaluating changes in fibrosis, although it is a relatively safe procedure when performed by experienced clinicians; it is an invasive procedure that has certain contraindications that can lead to complications.1 Diffusion-weighted imaging enables qualitative and quantitative assessment of tissue diffusivity. Random motion of water molecules in the liver can be quantified by calculation of the apparent diffusion coefficient (ADC).4
The ADC of livers with moderate or advanced fibrosis and cirrhosis has been reported to be lower than that of normal livers or livers with mild fibrosis across multiple studies, although reported ADC values vary between studies.1 A hurdle to the widespread clinical application of DWI lies in the lack of standardization of parameters. The variability in reported ADC measurements is further complicated by the use of different b values and acquisition methods based on breath-hold, free-breathing, or respiratory triggered techniques, which can affect ADC quantification.5
Subjects
This study included one hundred (100) patients with history of chronic hepatitis C [80 male, 20 female; where the age ranges from 26 to 57 with mean age (41.6+9.3) and a male to female ratio (4:1)] and twenty five (25) healthy volunteers [13 male, 12 female; where the age ranges from 23 to 55 with mean age (42.4+11.8) and a male to female ratio (1.08:1)].
Inclusion criteria included patients with chronic hepatitis C, based upon pertinent clinical history and results of liver function tests, who were referred from the hepatology clinic as a protocol for their antiviral treatment preparation, from July 2010 to March 2013. Twenty five (25) healthy adult volunteers with normal liver function tests, no history of hepatitis or diffuse liver disease, or abnormal liver imaging findings were included on the control group. The protocol was approved by our local institutional review board, and informed consent was obtained from all participants. Patients with absolute contraindications for MR imaging were excluded from both patients and control groups.
Diffusion-weighted MRI protocol
MRI techniques were performed in the diagnostic radiology department, MRI unit, using a 1.5 tesla super conducting unit (Philibs Achieva), where a quadrature phased-array multi-coil was used. This was done on the same day before liver biopsy was take; so we avoided any biopsy related complication artifact and also to standardize the time of delay between DW-MRI and biopsy between the patients which helps to avoid any change in the fibrosis stage and clinical picture of patients; either by long time of delay or treatment intervention, so reliable ADC measurement could be obtained.
The following parameters were used: TR/TE, 1.600-3.400/67-82; slice thickness, 8 mm; inter-slice gap, 1.6 mm; interleaved slice acquisition; field of view, up to 400 mm with 75-80% rectangular field of view; matrix size, up to 192 × 192; parallel imaging factor 2; 1-4 averages; b values of 0, 200, 500, 700 and 1000 s/mm². Multiple b-values were used to obtain an accurate quantitative analysis of diffusion-weighted images and consequently reliable ADC maps as well as the ADC measurement (because at low b-values, there is risk of perfusion contamination, so ADC measurement will be not reliable to assess diffusion of tissues by mixed effects of perfusion and diffusion that couldn't be separated at these levels while at high b-values, there is risk of noise contamination and bad images resolution with subsequent non- reliable ADC measurement). DWI was performed using a single-shot echo-planar imaging (EPI) fat-suppressed sequence in the axial plane, during a single end expiratory breath-hold.
ADC quantification
Using a commercial workstation (view forum workstation, Phillips Dicom), ADC maps were formed automatically then a single observer placed circular regions of interest (ROI) approximately 1-2 cm in diameter in four locations within the liver for each b value (same slide location), taking in consideration avoiding areas of artifact, GB, vessels, and focal lesions and another circular ROI was placed on the spleen at the same slide. The mean hepatic ADC was calculated as the arithmetic mean of the four ADC values calculated at each b-value.
Liver biopsy and histopathology
Percutaneous liver biopsy by true cut needle was done under ultrasound guidance for 91 patients who were previously diagnosed with chronic viral hepatitis; later histopathological assessment was done. Nine patients were not candidate for liver biopsy & were stratified as definite cirrhotic cases (F6 stage) according to clinical findings, laboratory & imaging criteria. Liver fibrosis staging was evaluated by an experienced hepato pathologist using modified Ishak scoring system and stratified as:
Statistical analysis
PSS version 14 was used for statistical computations. Liver and spleen ADCs were compared with Student’s t-test. Liver and spleen ADCs and normalized liver ADCs were compared between patients stratified by fibrosis stage using Student’s t-test test. Differences in ADC values were considered to be statistically significant when p < 0.05.
Liver ADCs were correlated with fibrosis scores using the Spearman’s rank correlation coefficient, while Receiver Operating Characteristic (ROC) curve analysis was used to determine the area under the ROC curve (AUC), and the threshold ADC was used to maximize the average of sensitivity and specificity and to provide the highest overall accuracy to distinguish between different stages of fibrosis.
The ADC of hepatic parenchyma was compared with that of healthy volunteers using Student’s t-test. Student’s t-test was also used for comparison of group parameters. The cut-off point was designated using Receiver Operating Characteristic curve (ROC) analysis. The results were considered significant if they had a P < 0.05 value. Validity of ADC for diagnosis of hepatic lesions was tested by sensitivity, specificity; the statistical analysis of data was done by using SPSS program (Figure 1 & 2).

Figure 1E Obtained at b value of 1000s/mm2 shows placement of regions of interest on liver parenchyma. Mean hepatic ADC at b-200s/mm2 was 1.89 x10-3 mm2/s, compared to mean hepatic ADC 1.22 x10-3 mm2/s at b-1000 s/mm2.
Figure 1 A 45 years old female with chronic hepatitis C virus (fibrosis stage 2 according to modified Ishak classification). Single end expiratory breath-hold axial single-shot echo-planar diffusion-weighted images obtained with increasing b values. A-D, b=200 s/mm2.
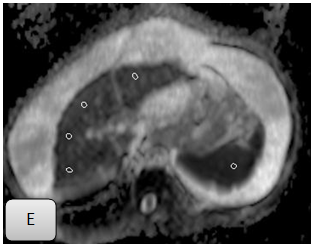
Figure 2E Obtained at b value of 1000s/mm2 shows placement of regions of interest on liver parenchyma. Mean hepatic ADC at b-200s/mm2 was 2.29x10-3mm2/s, compared to mean hepatic ADC 1.53x10-3 mm2/s at b-1000 s/mm2.
Figure 2 A 50 years old male with chronic hepatitis C virus (fibrosis stage 6, based on clinical, laboratory & imaging criteria). Single end expiratory breath-hold axial single-shot echo-planar diffusion-weighted images obtained with increasing b values. A-D, b=200s/mm2.
Results
Subjects and histopathological results
Ninety one patients in the case group performed ultrasound-guided liver biopsy and histopathological examination after doing the MRI diffusion weighted imaging. They were stratified according to modified Ishak scoring system into six stages (Figure 3), in which stage 2 represented forty percent of the whole study group, while the least was stage 5 represented by 4% only. The nine patients with well-established cirrhosis who did not undergo liver biopsy were considered “stage 6” fibrosis on the basis of clinical history, laboratory findings and confirmed by the MRI findings clearly showing liver cirrhosis according to established criteria.

Figure 3 The percentage of each liver fibrosis stage, according to modified Ishak scoring system among patient group.
ADC quantification and statistical analysis
There was a trend toward a decrease in hepatic ADC with increasing degree of fibrosis, where we found a negative correlation between mean hepatic ADC and fibrosis staging at which (r= -0.424, -0.558) with b-value 200 and 1000 s/mm2, respectively, with P=0.000 which is highly significant, while there was negative correlation at b= 700 s/mm2 which was statistically insignificant, also at b value= 500 s/mm2 there was positive correlation which was statistically insignificant (Table 1) (Figure 4).

Figure 4D Correlation between ADC 1000s/mm2 and liver fibrosis stages.
Figure 4 The correlation between fibrosis stages according to modified Ishak classification and mean hepatic ADC values at different b-values.
|
*ADC at b-value s∕mm2 |
Pearson Correlation |
P value |
|
200 |
-0.424 |
0 |
|
500 |
0.044 |
0.7 |
|
700 |
-0.105 |
0.3 |
|
1000 |
-0.558 |
0 |
Table 1 Correlation between ADC at each b value and stage of liver fibrosis
*Apparent Diffusion Coefficient (ADC)
*Significance level (P value)
*positive Pearson correlation was statistically insignificant
*P=0.000 was highly significant
By comparing mean hepatic ADC between individual stages of fibrosis at different b-values, there was a significant difference between control group and stage 1 at b-value 200 (p=0.000) and 700 (p =0.04) s/mm2 only, while on comparing mean hepatic ADC of non-cirrhotic versus cirrhotic patients at different b-values, there was significant differences between non-cirrhotic (stage≤4) and cirrhotic (stage≥5) patients at b-values 200(p=0.01), 500 (p=0.02) and 1000 (p=0.000)s/mm2 (Table 2). The difference of mean hepatic ADC between stages 1 and 2 was significant at b-value 200 (p=0.000) s/mm2 only, between stages 2and 3 at b-value 500 (p=0.01) and 1000 (p=0.01)s/mm2, between stages 3 and 4 at b-value 700 (p=0.04) and 1000 (p=0.03)s/mm2, between stage 4 and 5 at b-value 500 (p=0.01) and 700 (p=0.01) s/mm2, between stage 5 and 6 at b-value 200 (p=0.000) and 700 (p=0.000)s/mm2.
|
Fibrosis stage |
b-value (s/mm2) |
|||
|
200 |
500 |
700 |
1000 |
|
|
Control group |
1.9+0.52 |
1.5+0.2 |
1.4+0.09 |
1.3+0.08 |
|
Stage 1 |
2.7+0.8 |
1.6+0.1 |
1.5+0.2 |
1.3+0.1 |
|
P |
0 |
0.09 |
0.04 |
0.99 |
|
stages 1-4 (Non cirrhotic) |
2+ 0.6 |
1.5+ 0.3 |
1.4+0.2 |
1.3+0.1 |
|
stages 5,6 (Cirrhotic) |
1.5+0.8 |
1.7+0.08 |
1.4+0.2 |
1.1+0.06 |
|
P |
0.01 |
0.02 |
0.99 |
0 |
Table 2 mean hepatic ADC (value × 10-3 mm2/s) of the control group versus patients of the first stage of liver fibrosis as well as the non-cirrhotic patients versus cirrhotic stratified by fibrosis stage at different b values
*Apparent Diffusion Coefficients (ADC)
*Significance level (P value)
ROC analysis
Using the ROC curve analysis in our research to evaluate mean hepatic ADC ability to predict liver fibrosis stage ≥5 versus fibrosis stage ≤4 at different four b-values, we realized b-value 1000 s/mm2 to be the best value with combined higher sensitivity and specificity, where it shows area under the curve (AUC) 0 .879 with standard error 0 .035, confidence interval (95%) =0.811-0.947, significance level (P-value) 0.000 with 80.5% sensitivity and 77% specificity (Figure 5). At b-value =200 s/mm2, it shows area under the curve (AUC) 0.46 with standard error 0.091, confidence interval (95%) =0.463-0.82, significance level (P-value) 0.1 with 90.8% sensitivity and 46.2% specificity. On b-value =500 s/mm2, it shows area under the curve (AUC) 0.302 with standard error 0.051, confidence interval (95%) =0.202-0.401, significance level (P-value) 0.021 with 19.5% sensitivity and 77% specificity. On b-value =700 s/mm2, it shows area under the curve (AUC) 0.538 with standard error 0.102, confidence interval (95%) =0.339-0.738, significance level (P-value) 0.66 with 59.8%.

Figure 5 Receiver operating characteristic curve analysis; predictive ability of mean Hepatic ADC for liver fibrosis stages ≥5 versus liver fibrosis stages ≤4 (at b-value =1000s∕mm2). Area under curve is 0.879; Significance level is (P-value) 0.000, with sensitivity of 80.5%, specificity of 77% and cut off value 1.12 x 10-3mm2/s.
The ROC curve analysis at b-value =1000 s∕mm2, shows that the best cut off value of mean hepatic ADC is 1.12x10-3mm2/s, where above this value the patient belongs to the patient group with fibrosis stage ≤4 while below this value means that the patient belongs to the patient group with fibrosis stage >5 with 80.5% sensitivity and 77% specificity and this finding was highly significant (Figure 5).
HCV infection accounts for approximately 40% of all chronic liver disease, resulting in estimated 8000-10,000 deaths annually, and is the most frequent indication for liver transplantation. The early detection of fibrosis and cirrhosis has important clinical implications in these patients. Antiviral treatment of chronic HCV can eradicate the infection, increase patient survival and reduce the need for liver transplantation.1
In our study we attempted to determine the usefulness of using apparent diffusion coefficient (ADC) of liver parenchyma for determining the severity of liver fibrosis, moreover we assessed the value of using different b-values during our study aiming to highlight the best b-value with higher sensitivity and specificity. After statistical analysis, our results showed a negative correlation between the mean hepatic ADC and fibrosis staging at b-value 200 and 1000s/mm2, respectively, with P=0.000 indicating high significance, while there was negative correlation at b=700 which was statistically insignificant, Also at b value= 500 there was positive correlation which was statistically insignificant. Different studies showed negative correlation as well, however with different best b-values,6-8 where the best degree of negative correlation achieved was at b-value 800s/mm2,6 b-value 500s/mm27 and b-value 700s/mm2.7 Using the ROC curve analysis in our research to evaluate mean hepatic ADC ability to predict liver fibrosis stage ≥5 versus fibrosis stage ≤4 at different four b-values, we realized b-value 1000s/mm2 to be the best value with combined higher sensitivity and specificity in discriminating both categories, where it shows area under the curve (AUC) 0 .879 with standard error 0 .035, confidence interval (95%)=0.811-0.947, significance level (P-value) 0.000with 80.5%sensitivity and 77% specificity.
A technical point of difference between our study and other multiple studies is that we use multiple, variable b-values and we didn’t use low b-value in order to avoid any perfusion effect, in order to obtain accurate ADC measures. Low b-values were used in some studies including (0,150,250&400s/mm2),9 while others use a mix of high and low, similar to what we used, including (0,200,500&1000s/mm2).7 The multiple b values (low and high) enabled more precise calculation of ADC with less perfusion contamination and less regional ADC variation.4
Better degree of differentiation of individual fibrosis stages with mean hepatic ADC was observed in our study compared to the results of other study8 which evoked that; ADC can’t perform well in differentiating individual fibrosis stages, but, this could be due to limited number of patients with intermediate stages of liver fibrosis included in the other study. The results of several studies match our findings by reporting the ADC values of cirrhotic patients to be lower than those of non-cirrhotic patients or of healthy volunteers.10-12 We stated that at b-value =1000 s∕mm2, the best cut off value of mean hepatic ADC was 1.12×10-3mm2/s, where above this value the patient belongs to the patient group with fibrosis stage ≤4 while below this value the patient belongs to the patient group with fibrosis stage >5 with 80.5% sensitivity and 77% specificity. A study was done on 2009, where it showed a significant difference in the ADC values of non-fibrotic (0) and cirrhotic (4) patients, with an ADC value of 115×10-5 s/mm2, it was possible with a high degree of sensitivity (96.3%) and specificity (81.8%) to differentiate control groups from stage 4 fibrosis.
However, there was substantial overlap in the ADC values of 1 to 4. No cutoff ADC value could reasonably separate low-stage fibrosis from high-stage fibrosis.13 Our study had several limitations. First, because we were confined to patients with virus C hepatitis only, planning for their antiviral treatment, so no wide variation in degree of hepatic fibrosis was achieved. Second, biopsy was not performed on nine patients who had an imaging diagnosis of cirrhosis. Among the many difficulties with hepatic DWI is the lack of a standardized imaging protocol. Studies performed on different MRI scanners using different software for ADC maps result in different ADC values.13 So, future work is needed to assess a larger number of patients, obtain a standardized DWI protocol to allow for better correlation of different research work and comparison with newer methods of
perfusion MRI and MR Elastography.
So finally we conclude, DW-MRI can be used in assessing hepatic fibrosis especially for those patients who had contraindication for true cut biopsy and also for follow up of treatment response. It was observed that mean hepatic ADC measurement can perform well in differentiating stages ≤4 and ≥5 by appropriate sensitivity and specificity. We recommend DWI to be incorporated into a routine MRI protocol with the best b-value was 1000s/mm2.
I thank Dr Medhat Refaat and Dr Manal ElTohamy for general assistance in manuscript preparation and for mentoring and teaching me, Honey Bakry for her helpful contribution in statistical analysis and finally, but not least, Bakry El Sayed; the MRI technologist for sincere help in fulfilling the designed protocol.
Authors declare that there is no conflict of interest.

©2015 El, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.