Journal of
eISSN: 2373-633X


Case Report Volume 8 Issue 4
1Department of Radiation Oncology, Koc University, School of Medicine, Turkey
2Associate Professor, Department of Radiation Oncology, Koc University, Turkey
3Adjunct Associate Professor, Department of Radiation Oncology, University of Texas MD Anderson Cancer, USA
4Professor, Department of Radiation Oncology, Koc University, Turkey
5Adjunct Professor, Department of Radiation Oncology, University of Texas MD Anderson Cancer, USA
Correspondence:
Received: July 09, 2017 | Published: September 15, 2017
Citation: Sezen D, Bolukbasi Y, Tabak L, et al. Exacerbation of idiopathic pulmonary fibrosis triggered by stereotactic ablative body radiotherapy. J Cancer Prev Curr Res. 2017;8(4):323-325. DOI: 10.15406/jcpcr.2017.08.00288
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and life-threatening interstitial lung disease. The prognosis is poor, with median survival of approximately 3years.1,2 The main affected demographic is male patients age 50 or more.3,4
Our understanding of the pathologic mechansim of IPF is in evolution. Classically believed to be a chronic inflammatory process, currently IPF is believed to represent the endpoint of a chronic fibrotic response initiated by abnormally activated alveolar epithelial cells.5,6 The clinical manifestations of IPF are characterized by chronic respiratory insufficiency punctuated by acute exacerbations. The acute exacerbations can be life threatening carrying approximately 70-80% mortality.7,8 The diagnosis of an acute exacerbation is based on five criterias: Previous or concurrent diagnosis of IPF, unexplained development of worsening of dyspnea within 30 days, radiologic pattern consistent with AE, no evidence of pulmonary infection and exclusion of alternative causes of acute lung injury.9
There is ongoing controversy regarding a possible association between IPF and lung cancer.10,11 When patients with IPF develop lung cancer, their limited pulmonary reserve challenges the available treatment options. Furthermore, these patients often cannot tolerate a pathologic diagnosis of IPF and we depend on clinical and radiologic diagnosis. To illustrate these concepts, herein we present a patient with history of IPF lung cancer and Stereotactic Body Radiation Therapy (SBRT) complicated by acute exacerbation of IPF. The clinical vignette and available literature are reviewed.
A 72year-old male patient visited the outpatient’s clinic complaining of progressive shortness of his breath. A suspicious opacity was detected on his chest X-ray and contrast- enhanced computed tomography of the chest revealed a 3.0x4.0x3.5 cm mass in the upper left lung. The tomography also identified subplevral honeycombing, traction bronchiectasis and reticular opacities. His clinical and radiologic findings were consistent with IPF. There was no endobroncheal lesion indicated in the fiberoptic bronchoscopy. Transthoracic fine-needle aspiration biopsy confirmed squamous cell lung cancer. For further staging, 18 F-2-fluoro-2-deoxy-d-glucose positron emission tomography and computed tomography (FDG PET/CT) was completed. Metabolic findings were consistent with the known primary tumor without any nodal or metastatic disease. The disease was classified as T2aN0M0 non-small cell lung cancer according to 8th edition of the UICC/AJCC TNM staging system. He also had a history of larynx cancer and vocal cord radiotherapy, chronic obstructive lung disease and arthritis. Due to his complex past medical history and poor performance status, the patient was regarded as medically inoperable. He was also not a good candidate for chemotherapy. Therefore, only definitive radiotherapy was recommended by the multidisciplinary oncology team. Increased risk of the potential side effects depending on radiation treatment especially in the presence of underlying lung disease was explained to the patient comprehensively, and his written informed consent was taken. Radiotherapy dose planning was based on contrast-enhanced 4D-CT (respiratory data sets are ‘binned’ by phase:0-100 % at 10% interval) images fused with FDG-PET/CT. Respiratory corralated imaging (3mm slice thickness) was generated by AcQSim CT simulation of Philips Brillance Big Bore CT, planning was performed by Pinnacle radiation therapy planning system. PTV was integrated tumor volume plus 5 mm. Lung parenchyme was delineated at 50% phase in order to consider the lowest healty lung volume. The prescribed dose was 70 Gy in ten fractions. Treatment plan was generated with six fields (330, 0, 30, 60, 120, 150 gantry degrees) and step and shoot tecnique with 100 segmentations. Mean lung dose was 4,5 Gy and V5, V10, V20 for total lung were 15% , 10% and 9% respectively. For ipsilateral lung, V20= 19%, V10= 22% V5=25% and mean dose was 9,6 Gy. Mean heart dose was 0.3 Gy, maximum dose was 0,8 Gy and also V40 was 0. One fourth of esophagus was within the treatment volume and V50, maximum dose and mean dose were 0, 13 Gy and 2,3 Gy, respectively. The patient completed the radiotherapy at Trilogy (Varian Medical Systems, United States) without any complications (Figure 1).
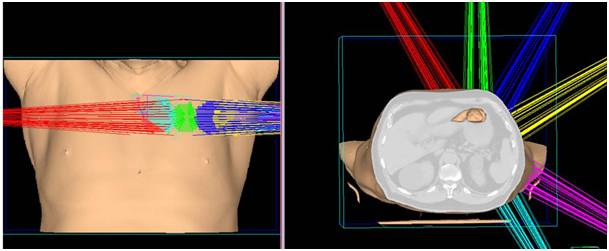
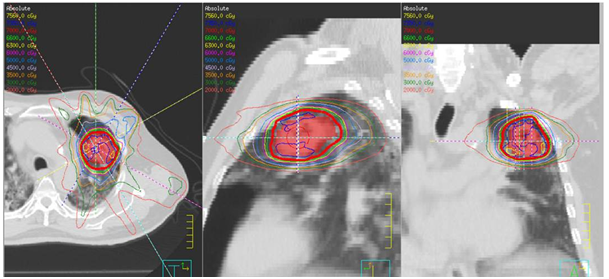
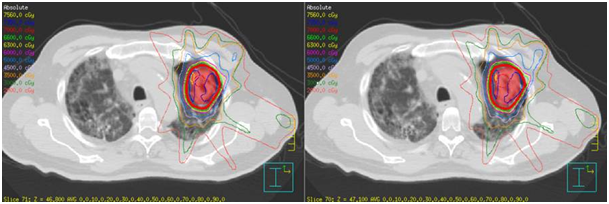
Figure 1 A) SABR treatment fields in 3 dimensions defining the left side treatment fields.
B) Axial, sagittal, coronal slides of treatment field of SABR treatment.
C) Poor lung parenchyma even at the time of SABR.
At the second week after completion of radiotherapy, he was emergently attended the pulmonary medicine clinic with rapidly progressive dyspnea and severe respiratory distress. Partial oxygen tension was 77 mmHg under 55% O2, and the chest X-ray revealed newly developing diffuse pulmonary infiltrates. Acute radiation pneumonitis was ruled out due to the fact that diffuse pulmonary infiltration was not matching the irradiated lung volume and radiotherapy entrance tracks; the contralateral right lung was mainly diffusely effected which was receiving insignificant radiotherapy doses (Figure 2). He had no fever or productive cough. The bronchial lavage bacterial and fungal culture findings were negative. Acute cardiac events were excluded with transesophageal echocardiography and electrocardiography. According to these data, the patient was evaluated as acute exacerbation of IPF and he was given prednisolone 160mg per day with meropenem 3x 500mg intravenous. In spite of using intensive medication his medical condition got worse. He was mechanically ventilated because of refractory hypoxia and he died of respiratory arrest on 15th day of the hospitalization.
Improvements in radiation therapy enable treatment of cancer patients with perfect dose distributions and minimal side-effects. Stereotactic ablative body radiotherapy (SABR) is also an effective and highly-focused treatment modality that has been widely used for several years. SABR, has high tumor control rates especially in medically inoperable patients with early stage non-small cell lung cancer.12 Nevertheless; in certain patient groups, as in this IPF case, side effects may be unavoidable despite advanced treatment modalities caring for treatment related pulmonary toxicity in treatment field.
Patients with IPF may have acute deteriorations during their clinical processes because of infections, pulmonary embolism, pneumothorax or heart failure. When this process presents without any identifiable cause, it is described as ‘acute exacerbation (AE)’.9 Pulmonary infection may be in the differential diagnosis of AE of IPF in present case. However, microbiological studies and results of blood tests were not consistent with any infection. Additionally, the patient had not fever or productive cough at the attendence to hospital and no response to prophylactic broad-spectrum antibiotics.
Radiation pneumonitis (RP) may be thought as the cause of these clinical and radiological symptoms especially due to the chronic damage of lung tissue. There have been few studies about SABR and its pulmonary effects. Radiation induced pneumonitis was reported as the most frequent acute pulmonary toxicity after SABR.13 Guckenberger et al investigated pulmonary injury after SABR and grade 2 and 3 pneumonitis rate was reported as 5.8 % with this study.14 Similarly, Yamaguchi et al. evaluated the association between subclinical interstitial lung disease (ILD) and fatal RP in patients with thoracic tumors treated with thoracic radiotherapy.15 According to this study, subclinical ILD was found as a statistically significant factor influencing the development of RP and fatal RP was reported more common in this group. In addition, extensive fibrosis on pre-treatment CT was regarded as a contraindication for thoracic radiotherapy. In the present case underlying lung disease had an important risk factor for RP. However, occurrence time and localization of lesions were not compatible with acute radiation pneumonitis, as diffuse pulmonary infiltration observed in X-rays were not matching the irradiated lung volume and the contralateral right lung was main site contributing the AE. Radiation related consolidation areas of pneumonitis needs to be within and adjacent to the radiation port;16,17 moreover, the timing of AE in our case is not fitting the usual RP generally developing between 3 to 5months after completion of radiotherapy. In particular, if patients with underlying pre-existing IPF develop RP after SABR, in majority of the patients radiologic changes in lung tissue do not arise before 3months after SABR and clinical symptoms of RP usually occur between 3 to 6months after SABR.18 Obviously, although RP was the main differential diagnosis for our case, the onset of the clinical and radiologic symptoms was not compatible with this.
The radiation dose for normal lung tissue might be another question of debate for the present case. In this context, Baker et al. evaluated the clinical and dosimetric predictors of RP in a large patient group treated with SABR to the lung.19 They reported that V20 of less than 10% is achievable and with this radiation dose RP is not statistically predictable. Additionally it was noted that if mean lung dose may be approximately 5-6 Gy, RP is not expected. In our case, V20 was 9%, and mean lung dose was 4,5 Gy. Furthermore, the other dosimetric parameters were consistent with the literature.
As described previously, there is conflicting data about SABR and its effects on lung tissue. But, in regards to clinical and radiologic occurrence time and radiologic pattern, acute exacerbation of IPF was thought to be the mainspring in this case. Furthermore, when the patient was reviewed; five criteria for AE of IPF were provided. He had a previous diagnosis of IPF and unexplained development of worsening of dyspnea within 30 days. While radiologic findings supported the diagnosis, alternative causes of acute lung injury were excluded. Lastly, there was no evidence of pulmonary infection by bronchoalveolar lavage.9 The SABR treatment here sounds to act as a possible trauma triggering the AE without correlation with irradiated field.
The use of proton therapy may be considered in such patients that have lung cancer with IPF. Because of the lower entrance dose and absence of exit dose of the proton beams, they have lower risk of complications compared to X-rays. Thus, unnecessary irradiation of mediastinum and contralateral lung can be avoided.20 However, Nagano et al. also reported a case that had acute exacerbation of IPF after lung cancer treatment by proton therapy.21 Recently, Ono et al. reported their clinical results of proton beam therapy in 16 patients with idiopathic pulmonary fibrosis.22 The present study revealed 20% rate of pneumonitis, including one case of grade 5 pneumonitis even with proton beam therapy. Depending on this reports; AE development cannot be directly associated with photon beams and exit doses, in the treatment of lung cancer patients with underlying pre-existing IPF.
Considering the high complication rate of all diagnostic and curative modalities in lung cancer patients with IPF, treatment remains complex and challenging. Even with highly efficient and safe treatment modalities such as SABR, we need to keep in mind possible AE in our IPF cases and should mention in our informed consent discussions that AE of IPF could be unavoidable though all measures are taken in planning and delivery of treatment.
None.
The authors declare that they have no conflict of interest.

©2017 Sezen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.