Journal of
eISSN: 2373-633X


Review Article Volume 13 Issue 4
Department of Radiology, King Khalid Hospital, Kingdom of Saudi Arabia
Correspondence: Essa Alhashlan, Department of Radiology, King Khalid Hospital, Kingdom of Saudi Arabia
Received: July 25, 2022 | Published: August 8, 2022
Citation: Alhashlan E, Alqufayli S, Almonajem MF, et al. Evaluation of female pelvic mass by MRI and CT scan. J Cancer Prev Curr Res. 2022;13(4):110-116. DOI: 10.15406/jcpcr.2022.13.00498
The human body consists of many parts with numerous organs, and there are multiple diseases that can potentially attack these organs. One of these vital body parts is the pelvis, which houses reproductive organs that are different according to gender. Because females have a reproductive system that experiences physiological changes according to age, benign or malignant masses may present in the pelvic area. Several types of masses unfortunately attack one organ called the ovary. Scientists claim that more than 125,000 people worldwide die annually due to ovarian cancer.1 There are, however, several methods used to obtain an accurate diagnosis, which therefore allows for effective treatment to take place. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are widely used in order to evaluate and stage masses within the ovaries. A critical question, however, is what the best radiology modality is in order to evaluate and identify the masses easily and accurately. This project aims to compare the radiology modalities (Magnetic Resonance Imaging (MRI) and Computed Tomography Scan (CT)) that could possibly be used to evaluate ovarian masses in the menstrual cycle stages. This paper will briefly discuss the gross anatomy and physiology of the ovaries. The radiological anatomy will also be mentioned, as will the pathology associated with the relevant organs.
The female pelvis contains several organs. A thorough understanding of the anatomy of the pelvic region is crucial for the diagnosis of any disease that could attack the female pelvis-and in order to determine treatment. For this reason, therefore, here is a gross anatomy of the pelvis, with a particular focus on the ovaries. The organs under focus are the reproductive system organs, which are: two fallopian tubes, two ovaries, uterus, endometrium, myometrium, cervix and vagina. Of particular interest is the middle compartment, which is found only in the female pelvis, while the anterior and posterior compartments are located in both genders. The middle compartment contains the female genital organs, which are: the ovaries, uterine tubes, uterus and vagina. This article will have a specific focus on the ovaries.
Ovaries are the female reproductive organs. The pair is located at each side of the uterus (Figure 1) and rest in the ovarian fossa. They are close to the lateral aspects of the pelvis.2 The ovaries are small, oval, greyish in colour and have an uneven surface. Their size is dependent on the female age and hormone status. However, they are around 3-5cm in length during childhood and become smaller during the menopause stage.3 Hamm B, et al.,4 in their ebook entitled “MRI and CT of the female pelvis” claim that adult female ovaries are 3-5cm long, 1.5-3cm wide and have a thickness of 0.5-1.Scm. At the beginning of the reproductive stage the surface is smooth, becoming increasingly irregular as the female ages. Within their capsule, ovaries comprise the following components: the ovarian stroma (it has fibroblasts, a smooth muscle cell, veins, arteries, nerves, lymphatics and follicles).4 A double fold of peritoneum is used to suspend them. Ureter and internal iliac vessels besides the origin of the uterine artery are located behind the ovarian fossa.2
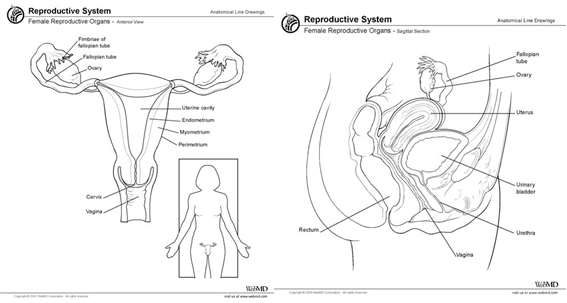
Figure 1 Female pelvis. Left: anterior section of the female pelvis. Right: sagittal section of the female pelvis.9
Ovaries have their own blood vessels feeding them, which are called ovarian arteries. The arteries are directly attached to the aorta in the abdomen and the arteries in the uterine through the ovarian branch of the uterine artery.5 The ovarian veins, on the other hand, begin from the venous plexus in the broad ligament. It is communicated with the uterine plexus. The two ovaries drain in different ways. The left vein drains into the left renal vein, while the other on the right side drains directly into the inferior vena cava.6
The nerve supply runs with the vasculature via the suspensory ligament of the ovary, and enters the ovary at the hilum. Supply is through the ovarian, aortic plexuses and hypogastric. In terms of lymph drainage, they drain into the aortic nodes and the iliac nodes.3
The ovary’s functional part is the follicles. Every follicle consists of an oocyte that is covered by one layer of somatic granulosa as a minimum, however, this can sometimes be numerous layers. There is a basement membrane that acts as a separation tool between the follicle and the surrounding stroma. There is no blood vessel supply to the granulosa cells.7 The follicles have two functions:
CT scan at a glance
CT combines x-ray and a computer to do tomography of the internal organs as slices in an axial projection. The dose is relatively high and radiation protection procedures should be used to shield the patient from radiation, which is carcinogenic.10 Now a days, mutislice CT scanners with a spiral option provide two things besides speed. Minimization of the motion artifacts and thinner slices allow for the detection of very small masses and facilitate volume imaging. Therefore, multi-planner imaging reconstruction is easily achieved. Also, the image will have high spatial resolution.4
In terms of positioning a patient, the patient must wear the hospital gown and remove all metallic, silicate or glass material to avoid artifacts. The patient should be in a comfortable position (on a soft surface, head supported and hands above the head, a cushion placed under the knees, and covered by a protective sheet) throughout the duration of the CT scan. The patient’s head will be first getting in the CT gantry, just in case of asking to admit a rectal contrast media to the patient. This position has some disadvantages in that: 1) the patient could move her head due to claustrophobia, 2) the intravenous line will be behind the CT gantry potentially trapping the patient withdraw from the gantry and 3) the communication between the staff and the patient will be from behind the gantry or through use of an attached microphone and speakers from the control panel. Feet first is an alternative position that has several advantages: 1) avoiding the feeling of claustrophobia for the patient, 2) the intravenous line will be at the front of the CT gantry and can be easily disconnected in case of withdrawing the patient from the CT and 3) communication is more easily facilitated with the patient.4
There are two types of contrast media that could be used in the CT scan of the pelvis. The first is orally and/or rectal admitted contrast media, which are barium sulfate and iodinated, aqueous solution. In the case of oral administration, the patient should have one-liter of contrast agent an hour prior to the CT examination. In the case of rectal administration, the contrast will be given on the table of the CT scan. Both techniques should be done after the enema. The second type of contrast media is intravenous contrast media that is nonionic, iodine-based. A dose of the contrast agent from 90m1 to l20ml with a concentration of 300mg to 370mg iodine/ml is recommended.4 It is also recommended that there is a delay of one minute after injecting the contrast agent to allow the contrast to reach the iliac, internal, external and common veins to exclude any venous thrombosis. The slice thickness should be from 3-5mm because a thinner slice will result in greater levels of noise in the images. A summary table is attached.4
Summary table of CT scan procedure for the pelvis4
MRI at a glance
On the day of the MRI examination, the patient must answer a safety question sheet that covers contraindications to MRI or to intravenous contrast media. The patient must be asked if she suffers from claustrophobia and be informed that a sedative will be given-as well as its potential side effects. Consent must be signed by the patient showing that the patient clearly understands the MRI procedures besides the contraindication of any metallic material. The patient must wear the hospital gown and remove all metallic, silicate or glass material to avoid artifacts. The patient should be in a comfortable position (on a soft surface, with head supported and hands above the head, a cushion under the knees, and covered by a protective sheet) at all times during the MRI scan. To avoid Peripheral Nerve Stimulation and possible burning in the attached organs like fingers, the patient must be protected by a sheet to ensure the bore of the magnet is not touched. The patient must also keep her legs and hands uncrossed.4
There are several methods that could be used to minimize the motion artifact during the MR procedures. These factors are located under the patient preparation because some of them need to be done hours prior to examination. Fasting for six hours or more is highly recommended to decrease the motion artifact from the internal organs. An antispasmodic drug should be given to decrease the motion artifact, and urination before the MR examination definitely decreases the displacement of the bladder on the pelvic organs subsequently resulting in less motion. Artifacts from the abdominal wall can be minimized as well by using a breathing trigger and gating. Additionally, a recommended technique would be to bind the receiver coil over the body to avoid excessive abdominal motion.11 Coils almost are phased array that enhances the SNR when compared with the conventional body coil. This allows the MRI operator to have either thin slices or fast sequences. However, presaturation must be applied if one uses the body phased array coils to reduce the motion artifact from the anterior organs.4
MRI protocols may start with a TI weighted image with these parameters: Sequence: TSE, TR = 400 to 600ms, TE = 10ms, NEX = 4 and Echo Train = 3. In order to differentiate between blood and fat, a fat suppression done with a combination of T1 is recommended. If it is time critical, T1 weighted image by GRE sequence (for example FLASH) is sufficient to acquire from 5 to 25 slices per breath-hold.4 To eliminate the motion artifact caused by involuntary movements, it is useful to use a technique called T1 weighted image with a RADAR-FSE sequence. It is radial acquisition regime-fast spin echo, which relay on the radial scanning where the radial scanning is defined as attracting increasing attention as a method of motion artifact suppression. This sequence suppresses the motion artifacts better than conventional FSE (figure 2). However, there are two downsides to this sequence, which are diminished sharpness and streak artifacts.12 Fat saturation T1 weighted image (figure 8,c) is also used to differentiate between the lesions in order to find out if the lesion has fat or not.13
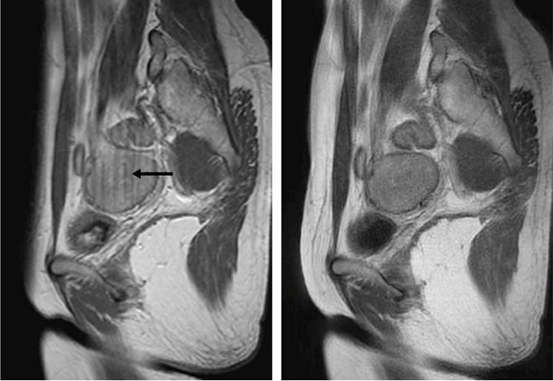
Figure 2 Left: T1 weighted image acquired by FSE and the black arrow shows the motion artifact. Right: T1 weighted image acquired by RADAR-FSE, which is free of the artifact.12
Contrast-enhanced T1 weighted image uses gadolinium, which is useful in order to evaluate complex lesions beside its ability to differentiate papillary projections or solid components from debris and clots. The amount of contrast media is calculated to be 0.1 mmol of gadolinium for every kilogram of body weight. It is recommended that the contrast be admitted via automatic injector because it enables the images to be acquired at multiple phases of enhancement by contrast medium. Examples include taking images before administering the contrast, and images taken 1 minute and 5 minutes after administering the contrast agent.13
T2 weighted image that uses TSE or FSE sequence is either conventional or single-shot. In the former sequence, the imaging procedure is achieved within 2 to 5 minutes while the patient is freely breathing. In the latter sequence the imaging procedure could be achieved within 15 to 20 seconds while the patient is holding their breath. Fat suppression, however, is not compulsory for use in the T2 weighted image sequence and can be nulled by using a technique known as STIR.4 T2 weighted images taken by a high magnetic field (7T) shows that the ovaries are better visualized than ones taken by 1.5T which evidence that as the magnetic field increases, so too does the image resolution.14
Diffusion-weighted imaging (DWI) is the ability of an MR signal to detect the random motion of the molecules. One of the advantages of using DWI is the provision of tissue contrast that is based on the diffusion of the molecules, which is in fact different to the contrast gained from TI and T2 weighted images. One could be interested in the intensity provided by DWI in order to diagnose tumors because this technique is not only used in neuroscience imaging. Despite the fact that the DWI has limitations in areas such as the pelvis where motion from internal organs occurs and causes artifacts because DWI is very sensitive to susceptibility artifacts, it can be acquired by a technique called SENSE. SENSE is a parallel imaging technique with sensitive encoding that reduces the phase encoding step to enhance image quality without affecting the spatial resolution. DWI also allows the quantitative evaluation of ADC, apparent diffusion coefficient, of the motion within the intravoxel. ADC allows differentiating between malignant and benign masses (Figure 3).15

Figure 3 These images show ovarian cancer in a 77-year old. a) Sagittal, T2 weighted b) Diffusion weighted image (b- va1ue=500) in the same sagittal shows increase MR signal, c) Diffusion weighted image (b- va1ue=1000) in the same sagittal demonstrate increase in MR signal, d) Fusion of a diffusion weighted image in the same sagittal and a T2 weighted image aids in anatomic acknowledgment of emphases increasing of MR signal intensity on DWI.15
Magnetic Resonance Spectroscopy (MRS) is an alternative MR procedure that provides limited data about the lesions. It is found that malignant tumors have an intense lipid peak while it is missing in the epithelial ones in spite of noticing the overlap with benign teratomas. In benign lesions, the lactate, choline or choline/creatine ration peaks are missing.13
Ovaries are easily identified through MRI and CT because of their situation, in addition to their soft tissue characteristics. The ovaries are characterized by a follicular structure that can be recognized on T2 weighted image on MRI. Using CT, they can be shown after opacities the bowl by contrast agent.4
On a CT scan, the two ovaries can be best identified after administrating bowel contrast. They are oval in shape, comprising soft tissue with low attenuation zones that represent normal follicles. The appearance of a dominant follicle, which has a range of one cm or more in size, facilitates the identification of the ovaries (Figure 4A & 4B). A useful method for detecting the hemorrhagic corpus luteum cysts in a CT scan is the identification of extreme attenuation values or the level of fluid-fluid.4
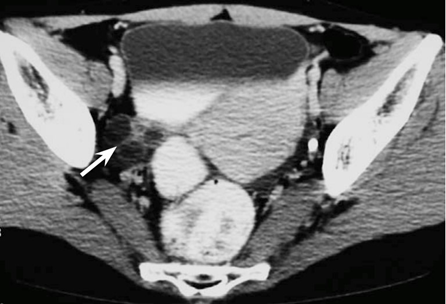
Figure 4A Shows the ovary in the right side (arrow), which is located in the ovarian fossa.4
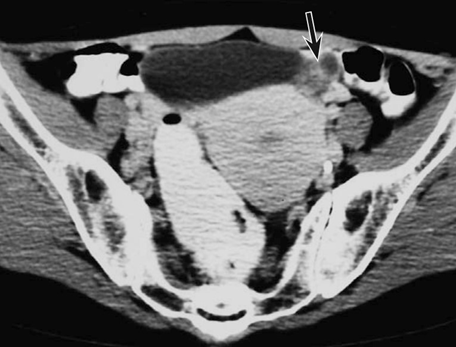
Figure 4B The left ovary located anterior to the uterine corpus, which is near the front abdominal wall (black arrow).4
Using MRI, the vast majority (95%) of women’s ovaries in the premenopausal stage can easily be shown by presenting the follicles within the ovary (Figure 5). On T1 weighted image, the ovaries are low to intermediate intensity of the signal (Figure 6, Left). In T2 weighted image, however, 70% of the ovaries have a zonal differentiation in the signal intensity from the medulla, which has a high signal intensity, and the cortex, which has a low signal intensity (Figure 6, Right).4 As the stroma of the ovaries remains somewhat low intensity of the signal, the follicular structure, on the other hand, can be discriminated easily on T2 weighted image.4 Ovaries are shown as homogeneous low to intermediate intensity on T1 weighted image while the follicles in the ovaries become hyperintense when compared with the surrounding stroma. The size of immature follicles is about one cm, while those in normal ovaries are closer to 3cm. In post-menopausal females, the ovaries look solid with a relative rise in stromal tissue that hypointense in T2. Also, they might contain minor T2 hyperintense follicles, with respect to the dysfunctional cysts that may be encountered if they are up to numerous centimeters in size. In addition to the ovaries’ size and presence, the follicles also depend on the age of the patient and the menopausal status.13
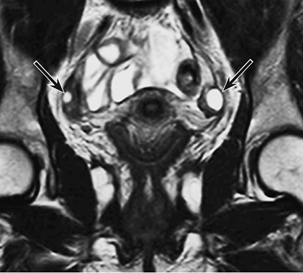
Figure 5 T2 weighted image of the follicles in the ovaries of a 28-year-old woman, which displays a very high MR signal on the T2 weighted image.4
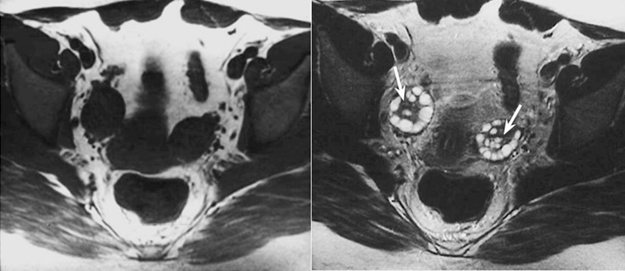
Figure 6 In the T1 weighted image (left), the ovaries are low to intermediate intensity of the signal, while in T2 weighted image (right), there is a zonal differentiation in the signal intensity from the medulla which has high signal intensity, and the cortex which has low signal intensity.4
Pathology of the pelvis
Pelvic masses, benign or malignant, can be described as a palpable mass arising from the pelvis on abdominal and/or vaginal examination. However, a pelvic mass is frequently diagnosed by a radiological examination.16 Pelvic cystic masses are common in females of all age groups. They are often asymptomatic and discovered at routine health visits, coincidentally found during a pelvis examination for another matter, or seen during the evaluation of a particular gynecological complaint.17 Most ovarian tumors and cysts presented are benign, round and well-circumscribed, with a cystic wall and near-water density that is hard to see. They are more common in menarcheal women.18 There are several types of benign and malignant masses. Here are some examples related to ovarian masses in general:
Functional cysts: These occur in the reproductive age period. They can present with acute pain either because of a haemorrhage into the cyst or torsion, and are seldom larger than 8 to 10 cm. Some, like corpus luteal cysts, are prone to rupture while others, such as theca lutein cysts, appear bilateral and occur during pregnancy.16
Benign neoplasms: There are subcategories for this division, which are: 1) Endometriotic Cysts, 2) Dermoid Cysts and 3) Serous and Mucinous Cystadenomas.
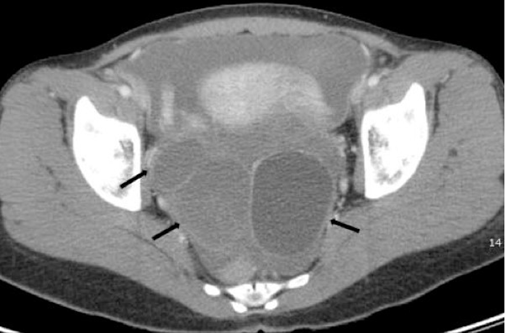
Figure 7 Three arrows pointing to endometriotic cysts in both ovaries in an axial projection of the pelvis in a CT scan image.19
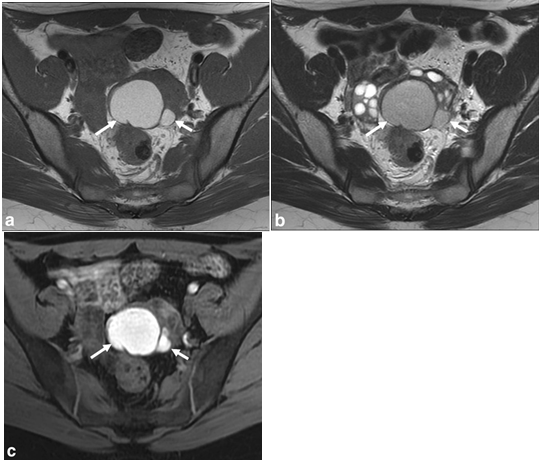
Figure 8 a: Axial T1WI shows two high signal intensity lesions appearing from the left ovary (arrows), 8b: T2-WI shows the lesions having lower signal related to T1 imaging, with“T2 shading” demonstrated, 8c: Fatsat-T1 image confirms that the lesions do not hold fat and in fact increase in signal intensity, consistent with endometriomas.13
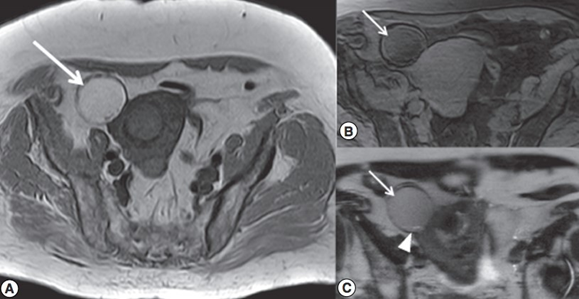
Figure 9 a: The arrow pointing to adnexal mass in the right ovary in an axial projection T1WI, b: Fatsat image shows that the fat signal is saturated, c: Two arrows pointing to dermoid cyst in T2WI.20
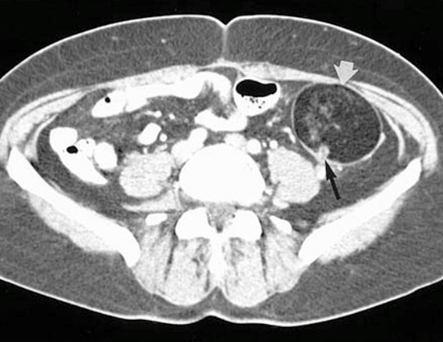
Figure 10 Enhanced CT for a pelvis shows the dermoid cyst (white arrow).21
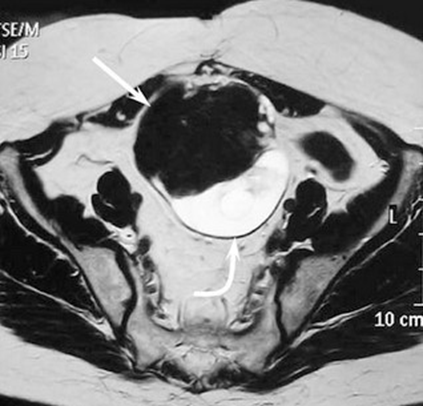
Figure 11 T2 WI shows a curved arrow pointing to a multilocular cystic lesion.22
On the other hand, malignant neoplasm affecting the ovaries can be classified as 1) Epithelial Cancers, 2) Non-Epithelial Cancers, 3) Germ Cell Tumors and 4) Sex cord-stromal Tumors.
Cross-section imaging techniques, CT and MRI, can afford staging evidence, which can be used to assist not only in surgical planning but also in treatment plan options.23
CT scan
CT scans with features such as helical or spiral modes allow the exam to be done in single shot with a breath-hold, which is in this case no more respiratory artifacts. This method is superior to the conventional CT because of the reasons mentioned and because of the limited radiation used to acquire three dimensional images.23
CT scans play a pivotal role in tumor detection and staging. In fact, CT scan accuracy in terms of staging is as high as 70% to 90%.23 In CT scans, the ovarian cysts need to be enhanced using a contrast agent (oral and intravenous contrast media). It is recommended that the contrast agent be given to the patient orally and via the rectum to differentiate the structures among the soft tissue, the adnexal and the bowel loops. The slice thickness should be a 5mm to 10mm axial section and the area covered should be from the area below the inferior pubic ramus to the iliac crest.23 CT demonstrates a cystic mass in the area of adnexal. It is characterized as well-defined borders with fluid density.18 In terms of ovarian neoplasia, a CT scan of the abdomen often demonstrates a complex mass that has alternating cystic and solid parts. There might be omental and cake coexistent ascites, signifying omental involvement by cancer. Benign neoplasm masses appear on CT scans as a cyst with either a unilocular thin-wall or multilocular thin-wall. It is usually performed to stage of neoplasia of the ovaries.24
MRI
In order to detect and characterize any lesion in the ovaries, MRI should be done in at least two orthogonal planes, with a routine MRI ovarian assessment ideally including axial and sagittal planes. The addition of the coronal plane, however, might assist in proving the result as well as helping the evaluation in the case of large ovarian masses that may be abdominally extended. T1 weighted image is necessary in order to determine the lesion characteristics. An example of that is establishing whether the mass within the ovary has hemorrhaged or contains fat. Contrast agents noticeably improve T1 weighted images and improve mass characterization. Using a contrast agent (Gadolinium-chelated) will enhance the TI weighted image, and therefore the detection of the omental and peritoneal implants will be enhanced in a patient with carcinoma in the ovaries. It is recommended that a bolus of contrast agent is given and the scanning start immediately, although a delay could help detect diseases such as fistulae.
T2 weighted images, on the other hand, have several features: 1) they help in differentiating between follicles and the bowel loop because the follicles have high signal intensity, 2) they help define the adnexal (figure 12) or uterine origin of a mass in the pelvis and 3) they help in staging and characterization of the lesion.23 Ovarian cysts are generally shown in TI weighted image as low signal intensity while in T2 weighted image they are seen as high signal intensity.18
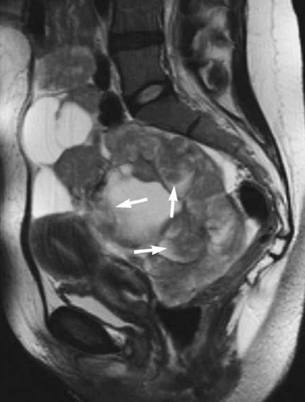
Figure 12 T2 weighted image shows a large adnexal mass.4
Comparison between CT and MRI
CT scans are known as the gold standard due to their widespread use in order to diagnose and stage masses in oncology, not to mention benefits arising from the ability to re-evaluate the mass staging after treatment. It does, however expose the patient to a high dose of radiation and also causes the differentiation process of radiation fibrosis from recurrent tumor is hardly to be defined. As MRI does not require a contrast agent and CT procedures do, MRI is superior, to what extend, to get sufficient data from the image.25
MRI has a coil called an endorectal, which allows the MRI to provide a high level of differentiation among tissues of all pelvic organs besides the high spatial resolution but in a small FOV.25
In addition to CT’s limited contrast resolution, it is hard to compare normal lymph nodes and the metastasis. Not only is the CT limited in this manner, but MRI cannot decremented the benign lymph nodes from malignant ones because they have same T1 and T2 relaxation times and proton densities. Both these imaging modalities rely on the size of the lymph nodes to identify which one has metastases MRI is superior to CT at the staging level of the pelvis mass.26 Ovaries in unusual locations within females of childbearing age can largely be identified because of the standard morphology of follicles. Ectopic or mal-descended ovaries can be easily identified using MRI, and it is superior to CT in this manner because the T2 weighted image is an excellent visualizer.4
It is recommended that if the ovarian masses are either benign or malignant and the masses are large and palpated, one would do a CT scan of the entire pelvis and possibly the abdomen to determine if the abdomen is experiencing any cancer metastases. CT is very good at evaluating lesions, however if the mass is small, MRI is superior to CT for detection and evaluation. In addition, MRI is superior to CT because of: 1) high spatial resolution with thin slice, 2) masses can be visualized in three different projections, and 3) no ionized radiation will be emitted to a patient.
This report touched on the anatomy, physiology and pathology of ovarian masses experienced in females of childbearing age. It is shown that there are several types of lesion that can be classified under two categories: benign and malignant. The subcategories of benign lesions are: 1) endometriotic cysts, 2) dermoid cysts and 3) serous and mucinous cystadenomas. Malignant neoplasms affecting the ovaries, however, can be classified as: 1) epithelial cancers, 2) non-epithelial cancers, 3) germ cell tumors and 4) sex cord-stromal tumors. Imaging modality- CT scans and MRI - were also mentioned as they are usually used in evaluating and staging masses, although there are different types of imaging modality that can also evaluate or assist in diagnosing the ovarian lesions. MRI procedures are deemed superior to CT scans in order to investigate ovarian lesions either for staging or evaluating them.
None.
Authors declare that there is no conflict of interest.

©2022 Alhashlan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.