Journal of
eISSN: 2373-633X


Review Article Volume 10 Issue 5
1Purdue University, West Lafayette, USA
2Department of Electrical Engineering, College of Engineering, Anna University, India
3Arupadai Veedu Medical College & Hospital, Vinayaka Missions Research Institute, India
4KAP Viswanatham Government Medical College & Hospital, India
Correspondence: Raji Sundararajan, School of Engineering Technology, Purdue University, Rm 187, Knoy Hall of Technology, 401 N. Grant St. West Lafayette, IN 47907, USA
Received: October 05, 2019 | Published: October 25, 2019
Citation: Sundararajan R, Poompavai S, Sree GV, et al. Electro-therapies for sarcomas. J Cancer Prev Curr Res. 2019;10(5):119-124. DOI: 10.15406/jcpcr.2019.10.00403
Sarcomas are cancers that occur in soft tissues and bones that mostly affect teenagers and young adults. Although its occurrence is rare, the affect is huge-metastatic and deaths are imminent. This mini review reports the various aspects of sarcomas, including Ewing’s sarcoma and also discussed is the use of electrical pulse-based therapies, such as electrochemotherapy (ECT) and irreversible electroporation (IRE) for those patients who could not benefit from the standard therapies. Typically, 1200V/cm or 1000V/cm, 100us, eight pulses are used for ECT and bleomycin and cisplatin are the two most commonly administered drugs. Lately calcium and other bio-compounds, such as mint are explored as anticancer drugs. In IRE, 80 pulses of 1000-1500V/cm, 100µs are used. In general electrotherapies offer an attractive alternative to current standard therapies when they are refractive.
Keywords: sarcoma, Ewing sarcoma, soft tissue sarcoma, electrochemotherapy, irreversible electroporation
ECT, electrochemotherapy; IRE, irreversible electroporation; GIST, gastrointestinal stromal tumors; ESFT, Ewing’s sarcoma family of tumors; ES, Ewing sarcoma; PNET, peripheral primitive neuroectodermal tumor; NSE, neuron-specific enolase; EP, electroporation
Sarcomas are cancers of the bones and soft tissues.1,2 They are generally divided into soft tissue sarcomas and bone sarcomas based on their different mesenchymal origins and anatomical locations. They develop from cells that maintain the structure or cushion other organs in our bodies, including bone, cartilage, muscle, fat, and tendons. The different types of sarcomas are: a) Primary bone sarcomas, including Chondrosarcoma, Ewing Sarcoma, and Osteosarcoma. The various soft tissue sarcomas are: Angiosarcoma, Gastrointestinal Stromal Tumors (GIST), Kaposi Sarcoma, Leiomyosarcoma, Liposarcoma, Synovial Sarcoma, Undifferentiated Pleomorphic Sarcoma, Desmoid Fibromatosis and Pigmented Villonodular Synovitis.2 They are uncommon and make up only 1 percent of all adult cancers.1 However, in children and young adults of up to 30years age, its occurrence is higher; up to 3/100,000 happen and especially Ewing Sarcoma is experienced by teens. Since sarcomas are transported by blood, their spread is quicker and often secondaries are possible, compared to other cancers, such as carcinomas. About 80 percent of sarcomas begin in the body's soft tissues (cartilage, muscle, fat, and tendons). The other 20 percent arise in the bones. Medical news today1 indicates that 60% of sarcoma starts in an arm or leg, 30% in abdomen or torso and 10% in neck or head. The greatest numbers of bone tumors are metastatic and spread to lung, colon, or breast. Figure 1 shows different types of sarcomas including angiosarcoma, osteosarcoma, Ewing sarcoma, Chondrosarcoma, etc.3

Figure 1 Types of sarcoma.3
Ewing’s sarcoma is a high-grade osteolytic malignant neoplasm, described by James Ewing in 1921.4 It is the third most primary malignant bone tumor, after multiple myeloma and osteosarcoma. It forms from a certain kind of cell in bone or soft tissue and may be found in the bones of the legs, arms, feet, hands, chest, pelvis, and spine as shown in Figure 2.5 It is classified as a group of small round blue tumor cells as shown in Figure 3, which includes neuroblastoma, alveolar rhabdomyosarcoma and lymphoblastic lymphoma.6 The Ewing’s sarcoma family of tumors (ESFT) includes classic Ewing sarcoma (ES), Askin tumor, and peripheral primitive neuroectodermal tumor (PNET).
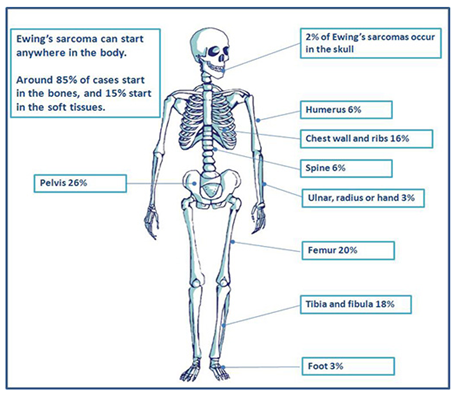
Figure 2 Occurrence of Ewing sarcoma in different parts of the body.5
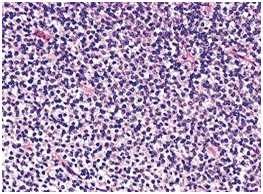
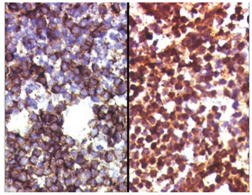
Figure 3 (A) Histology of Ewing sarcoma. (B) The tumor cells of EFT show membranous expression of CD99/MIC2, and (C) nuclear positivity for antibodies against FLI1.6
Morphologically, Ewing sarcoma is comprised of sheets of small round cells with a high nuclear to cytoplasmic ratio. The cells have weak eosinophilic cytoplasm containing glycogen and round nuclei with chromatin and little mitotic activity. Immunohistochemical analysis shows that 90% cases of Ewing sarcoma cells exhibit adhesion receptor CD99 or MIC2, usually related with the lymphoid cells mainly in leucocyte transmigration of the endothelium.7 Occasional rosette formation has been observed and does not produce any matrix. This tumor frequently undergoes necrosis and the residual viable cells show perivascular distribution. Rarely, these EFT tumor cells can be large with irregular nuclear membrane and prominent nucleoli.8
Antibody against FLI1, which is present in the center of the nucleus of the tumor cells has been shown to be specific for EFT.9 Tumor cells may also express neuron-specific enolase (NSE), synaptophysin, and S-100 protein depending upon the degree of neurectodermal differentiation.10
Sarcomas can be subdivided into two distinct classes, depending upon the genetic mutations. One class includes tumors having complex karyotypic abnormalities without a particular pattern and other class includes Ewing sarcoma comprises tumors with unique chromosomal translocations leading to specific fusion genes.11 Increasingly, Ewing family of tumors are being characterized by t(11;22)(q24;q12) chromosomal translocation , this generated fusion of 50 segment of the EWS gene with the 30 segment of the ETS family gene FLI-1. This resultant EWS-FLI-1 fusion protein acts as an aberrant transcriptional activator as shown in Figure 4, which contributes to the development of ESFT by altering the expression of its target genes in a better cellular environment.12 Table 1 shows the EWS fusion types in Ewings and other sarcomas.
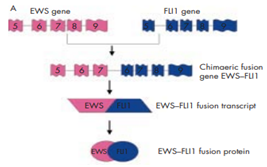
Figure 4 Schematic representation of the t (11;22) (q24;q12) translocation resulting in the generation of the EWS–FLI1 type 1 fusion transcript.
Translocation |
Gene fusion |
Tumour time (% of tumours with this EWS gene rearrangement) |
t[11;22](q24,q12] |
EWS-FLI1 |
ESFT (85%) |
t[21;22](q22,q12] |
EWS-ERG |
ESFT (10%) |
t[7;22](q22,q12] |
EWS-ETV1 |
ESFT (rare) |
t[17;22](q12,q12] |
EWS-E1AF |
ESFT (rare) |
t[2;22](q33,q12] |
EVVS-FEV |
ESFT (rare) |
t[12;22](q13,q12] |
EWS-AFT1 |
Clear cell sarcoma |
t[11;22](q13,q12] |
EWS-WT1 |
Desmoplastic small round cell tumor |
t[9;22](q22,q12] |
EWS-CHN |
Myxoid chondrosarcoma |
t[12;22](q13,q12] |
EWS-CHOP |
Myxoid liposarcoma |
Table 1 EWS fusion types described in Ewing’s sarcoma family of tumours and other sarcomas12
The cell plasma membrane human cells, is a microscopic bilayer of protein and phospholipids, with hydrophobic and hydrophilic ends.13 This structure allows only a controlled flow of selected ions and molecules through membrane proteins working as ion channels while regulating a concentration of ions on both of its sides (extra- and intra-cellular). This gives rise to a potential,14 which allows the cell membrane to function as a leaky dielectric, having both capacitive and conductive properties due to charge accumulation and conduction. In general, large, charged and polar chemotherapeutic molecules are impermeable or poorly permeable across the membrane, due to its structure.
The local application of short duration, high-intensity electrical pulses across the cell membrane results in the charge accumulation and increases the Vm. When the Vm increases beyond the threshold (>0.5V), there is a several-fold enhancement of electric field, leading to the pore formation.15,16 This phenomenon, known as electroporation (EP) can enhance the uptake of external molecules up to 1000 times.17 When EP is applied towards the uptake of chemotherapeutics, this non-surgical, physical procedure is called Electrochemotherapy (ECT).
ECT is gaining momentum as an alternate modality for advanced, inoperable, radiation and chemo-resistant tumors, including cutaneous and subcutaneous metastases in Europe.18 Electroporation generally depend upon the intensity of the electric field, the duration of each pulse, the number of pulses, and the interval between them. Depending upon the magnitudes of these parameters, we can have three effects on the cell membrane: no changes on the cell membrane, temporary opening of the cell membrane after which cell can still survive (reversible electroporation), and permanently open the cell membrane and the cell dies after (irreversible electroporation). Reversible electroporation is used in electrochemotherapy, wherein low dose chemodrugs and machomolecules are administered into the targeted area.19–23 Electrodes are placed around the tumor area, generating reversible permeabilization that allows substances to pass the cell membrane and into the cytoplasm. Irreversible electroporation (IRE) is that which causes permanent permeabilization of the cell membranes and the consequent loss of cell homeostasis, when an electrical field is applied to cancer cells.24–28 Advantages of IRE are that there are no drugs in this and it is a non-thermal mechanism of action, dependent on the blood flow, allows focal tissue ablation, and it requires a short time of application.13 IRE allows ablation of tumors in close proximity to other tissues. Other ablation mechanisms such as radiofrequency thermal ablation (RFA) release energy to the surrounding tissues and affect the vascularity and connective tissue structure.25 RFA first uses ultrasound, computed tomography, or magnetic resonance to locate the tumor and then insets a small needle-electrode into the tumor.29 Ionic vibration at the needle tip generates frictional heat that ablates the tumor. Electrotherapies could be novel treatment modalities to treat inoperable, recurrent sarcomas using bleomycin and electrical pulses.
ECT is extensively used in animal models of sarcoma,30–33 human treatments of STS patients limited.34,35 ECT was used also in Kaposi’s sarcoma.36,37 Campana et al.38 report the ECT treatment of 34 soft tissue sarcoma (STS) patients on locally advanced and metastatic soft tissue sarcomas. In this case, intravenous bleomycin (15,000 IU/m2) was used, followed by ECT. The pulse parameters used are: 1200V/cm, 100µs, 8 pulses at 5kHz frequency. The STS patients were refractory to or unable for conventional treatments due to either poor performance status or comorbidities. Szewczyk et al.39 report about using calcium electroporation for treatment of sarcoma in preclinical studies. In this case, they studied a normal murine muscle cell line (C2C12), and a human rhabdomyosarcoma cell line, in the undifferentiated and differentiated state. Electroporation was performed using 8 pulses at 600-1000V/cm using calcium as the chemo drug at concentrations of 0.5, 1 and 5mM. The results indicated that calcium electroporation was tolerated significantly better in normal muscle cells compared to sarcoma cells and this could be sued for local treatment of sarcoma.
In another case of a 24 year old male with recurrent sarcoma on his head (near ear), electrochemotherapy (ECT) was used as no accepted standard therapy was left.35 Using a MedPulser (Genetronics Inc., Sandiego, CA) and a circular 6 needle array electrode, the patient was treated under general anesthesia. Bleomycin was used for this treatment. The 50x35mm tumor and 0.5cm margin were treated with 63 overlapping applications. Patient had no pain complaints and after 10 weeks, no tumor was seen anymore. This is the first clinical report of treating sarcoma successfully using bleomycin-based ECT.
Irreversible electroporation is gaining momentum as a nonthermal tissue ablation technique in treating sarcomas.40,41 Steinbrecher et al.42 report about using IRE as a curative treatment for Ewing’s sarcoma. In this case, the patient, a 9 year old girl could not undergo surgery or radiation due to the high risk of sacral nerve plexus destruction and paralysis. Four cycles of salvage chemotherapy using vincristine, irinotecan, temozolomide and zoledronic did not reduce the tumor size. Since surgery and radiation is not possible in this patient as mentioned above due to the risk of neural damage and possible paralysis, and chemotherapy is not working, IRE was chosen as the best option for a curative paradigm. The patient went through IRE following the fifth cycle of chemotherapy. Using CT guidance and five 11-gauge coaxial trans-osseous needles, IRE was performed under CT guidance. Two IRE probes were used and the procedure was completed with no complications-there were no loss of lower extremity, bowel, or bladder motor strength or sensation. Follow-up using MRI and PET-CT showed a complete radiologic response. Three years later, patient remains in continuous clinical remission.
Both single electrode probe and two electrode probes (Figure 5) are used for IRE for treating a 7-year old spayed female Labrador retriever.43 It had a five year history of degenerative coxofemoral joint disease, causing bilateral pelvic limb lameness. The voltage applied varied from 1000 to 1500V, with the exception of one, where 800V was used. 80 pulses were applied each time, with a pulse length of 100µs, except in one case, where 70µs was used.
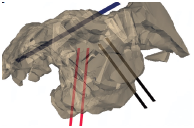
Figure 5 Single and two probe electrodes used for IRE.41
The main advantage of IRE in comparison to other ablation techniques are its non-thermal nature. Due to localization of the applied electric field structures such as collagen and elastin are not damaged due to the treatment. In addition, the electrical properties of cancer cells are more conducive to electrical pulse-based therapy than normal cells.44 The cancer cells are more conductive, has more sodium, and lower membrane potential-all make the cancer cells more susceptible to the electrical pulses and the electric field than the normal cells. In addition, since electric filed is inversely proportional to the inverse of distance squared, proximity effect is minimum or nil. The literature search indicates that this is a first treatment of ES using IRE.
None.
The authors declare there is no conflict of interest.

©2019 Sundararajan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.